Application of red mud as both neutralizer and catalyst in supercritical water oxidation (SCWO) disposal of sewage sludge
Hongzhen Chenabc,
Guangwei Wang*abc,
Yuanjian Xuac,
Zhong Chenac and
Fengjun Yinac
aEnvironmentally-Benign Chemical Process Research Center, Chongqing Institute of Green and Intelligent Technology (CIGIT), Chinese Academy of Sciences, No. 266 Fangzheng Avenue, Shuitu Hi-tech Industrial Park, Shuitu Town, Beibei District, Chongqing, 400714, P. R. China. E-mail: wangguangwei@cigit.ac.cn; Tel: +86 23 65935820
bKey Laboratory for Solid Waste Management and Environment Safety, Ministry of Education of China, Tsinghua University, Beijing, 100084, P. R. China
cKey Laboratory of Reservoir Aquatic Environment, Chinese Academy of Sciences, Chongqing, 400714, P. R. China
First published on 31st May 2016
Abstract
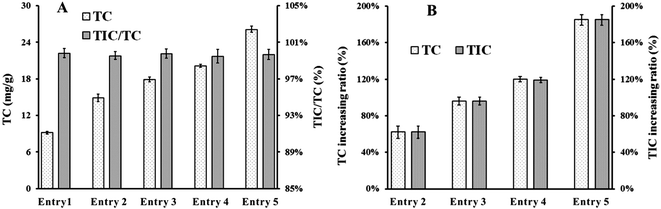
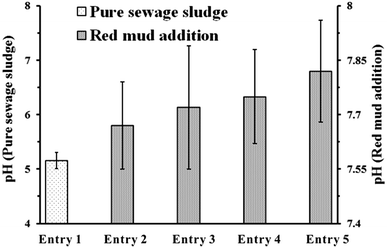
Red mud was used in the supercritical water oxidation (SCWO) disposal of sewage sludge, not only as a neutralizer for acidic substances produced in situ, but also as a catalyst for decomposition of pollutants. With initial amounts of 2, 4, 6, and 8% of red mud, the total carbon (TC) value of the solid residue increased from 9.16 mg g−1 to 14.88, 17.91, 20.11, and 26.14 mg g−1, respectively. This resulted from the net increase in the inorganic carbon after capturing and absorption of CO2 produced in situ. The pH of the drained water was correspondingly increased from 5.16 to the range 7.67–7.82. This was mainly determined by the balance in the system of soluble inorganic salts (SIS)–remaining organic substances (RS)–CO2–H2O under certain conditions. However, the pH controlling system switched to SIS–CO2–H2O and RS–CO2–H2O as the operating temperature increased and the residence time increased, respectively. An increase in the TOC removal rate was observed by increasing the amount of red mud, and an outcome in excess of 99.3% could be achieved, although at a relatively low temperature of 400 °C by an initial concentration of 8% red mud. Combined with the rise of TOC removal rate after 2% red mud addition confirmed in the experiments with different operating temperatures and that with different residence time, the catalytic effect of red mud was strongly evidenced. It was probably attributable to the active components in red mud at the primary period, and to the fine-grained calcite that formed in situ, along with tricalcium aluminate, perovskite, hematite, and other inorganic salts at the subsequent period.
1. Introduction
The quantity of sewage sludge to be handled has increased significantly due to the upgrade and expansion of wastewater treatment plants as industrialization and urbanization spread widely, and has become an important environmental issue, particularly in the cities of some developing countries.1,2 Incineration3,4 or co-incineration5,6 have been considered effective options for sewage sludge treatment; however, many individuals and organizations worry about the potential long-term risks of gaseous toxic compounds (e.g., dioxins)7–9 and fly ashes (regarded as an important reservoir of heavy metals)10,11 produced by these treatments. Therefore, the urgency of developing additional appropriate disposal routines with great treatment capacity, which could enable the treatment of sewage sludge without environmental concerns, is clearly recognized.Supercritical water oxidation (SCWO) disposal of sewage sludge presents a more satisfactory avoidance of toxic emissions and fly ash due to the overwhelming destruction of organic compounds under the typical operating conditions (450–600 °C, 24–28 MPa).12–14 Moreover, the supercritical water (SCW) also works as a non-polar solvent for the majority of organic substances and light gases, even though they are insoluble in ambient water.15 As a consequence, problematic organic pollutants can be completely oxidized in a homogenized fluid phase, with the constituent carbon atoms being mineralized to CO2, and the hetero-atoms including sulfur, chloride, and phosphorus, are eventually converted to their corresponding inorganic acids or salts. Since the 1980s, SCWO has been extensively investigated by not only researchers but also engineers, and has been demonstrated as one of the most promising terminal treatments for a variety of sludge types.16–19
However, for the commercialization of SCWO,20–22 three key obstacles had been recognized that strongly constrain its expanded application: (1) scale coating and build-up on reactor walls or processing surfaces occurs due to the extremely low solubility of inorganic salts in SCW media.23,24 (2) There is a rapid corrosion and failure of reaction vessels and pipelines arising from its' constant exposure to the aggressive hydrothermal environment.25,26 (3) There is a high cost to heat and sustain a SCWO process, to pressurize the oxygenating reagent, carrying gas, protecting or catalyzing materials.27,28
By timely isolation of the solids precipitated in situ from the reaction system, some effective engineering approaches, including novel reactor design and operating technique optimization, have been developed to solve the problem of scale build-up.29–31 However, corrosion and high cost still remain important issues in the SCWO process. The available approaches just allow the processing of wastes with corrosion resistance for limited periods of time.32,33 To reduce cost, application of catalysts such as precious metals or metal oxides (e.g., manganese oxide, chromium oxide, and vanadium oxide) to decrease the processing temperature and accelerate the oxidation rate in a SCWO process, has been investigated by numerous researchers.34–37
More than 70 million tons of red mud, a hazardous waste from the industrial production of aluminum, is produced annually during the Bayer processing of bauxite around the world.38,39 The storage of such a large amount of red mud is an important environmental concern because alkaline slurry and solutions usually seep from the landfill sites or pipelines into the ground or into the groundwater, which may raise the alkalinity of the environment.40 By using the high alkalinity (pH 13) associated with its chemical composition in oxides of alkali metals and alkaline-earth metals, red mud has been reported to be an inexpensive and effective neutralizer of acidic substances.41–43 In addition, red mud has been widely applied to catalyze chemical reactions such as hydrogenation, de-chlorination, and hydro-de-chlorination oxidation of hydrocarbons, due to the variety of its active components and its fine-grained nature.44,45
The purpose of this paper was to investigate the SCWO disposal of sewage sludge using red mud not only as a neutralizer of acidic substances but also as a catalyst for oxidation. Red mud was introduced into SCWO reaction vessels by mixing it with sewage sludge at different ratios. In the reaction zone, CO2 and other acidic substances produced in situ, were neutralized and converted to stable solid minerals (e.g., calcite) that created solid residues. The oxidation of organic pollutants was simultaneously catalyzed by active surfaces in the red mud and by the newly formed fine grains surrounding the ash particles. As a result, both the aggressive acidic environment and the treatment efficiency of SCWO disposal of sewage sludge were expected to be highly improved. Furthermore, such use would make it possible to ease environmental concern about red mud storage, which is regarded as a long term risk for all organisms living nearby.
2. Materials and methods
2.1 Sewage sludge
Active sewage sludge used in this work was supplied by Xiao Jiahe Wastewater Treatment Plant (Chongqing, China). Properties of the sewage sludge were obtained by the directly analysis of this sample and the results are presented in Table 1. In which, the water content was determined by measuring the weight loss before and after it was dried at 105 °C for 8 h. Total carbon (TC) and total inorganic carbon (TIC) of the dried sludge was determined using an Analytic Jena Multi N/C 3100 Analyzer (Germany). During measurement of the carbon content, oxygen gas was used as burning gas and helium gas was used as a carrier gas to provide an inert atmosphere. The operating temperatures of 1150 and 850 °C were set in the combustion and reduction tube, respectively. Content of nitrogen in the dried sewage sludge was determined by using an Elementar vario El cube (Germany), while that of phosphate, sulfate and chloride were determined by Ion Chromatography (ICS 1100, American) after digestion pretreatment. The ash content of the dried sludge was determined by recording the weight of the remaining portion of the sample after it had been burned in a muffle furnace at 575 °C for 8 h.| Active sewage sludge | Water content (% w/w) | 95.5 |
| Dried sewage sludge | Total carbon (TC) (mg g−1) | 123.5 |
| Total organic carbon (TOC) (mg g−1) | 103.2 | |
| Nitrogen (mg g−1) | 23.4 | |
| Phosphate (mg g−1) | 9.85 | |
| Sulfate (mg g−1) | 3.53 | |
| Chloride (mg g−1) | 6.13 | |
| Ash content (% w/w) | 45.0 |
2.2 Red mud
Fresh red mud was supplied by Chongqing Company, Aluminum Corporation of China, in the form of damp-dry powder. The red mud sample was dried at 105 °C in an oven for 8 h and then grounded in a mortar and pestle. Subsequently, it was sieved through an 80 μm sieve and stored in a vacuum desiccator until used. The particle size distribution of the red mud was determined using a Master Sizer 33370-45 (Malverns, UK), which indicated that 78% of the particles were <48 μm in size. The surface area was found to be 10.8 m2 g−1 using a BET surface area analyzer (Quantachrome AUTOSORB-1, USA). The TC of the dried red mud was 824 mg kg−1, with a TIC/TC of 98.86%, which was determined in the same way as for the sewage sludge. The major component compounds of the red mud were determined using a SHIMADZU X-ray Fluorescence Spectrometer-1800, and are shown in Table 2.| Major oxides | CaO | SiO2 | Fe2O3 | TiO2 | Al2O3 | MgO | Na2O | K2O |
|---|---|---|---|---|---|---|---|---|
| Composition% w/w | 44.69 | 27.99 | 11.60 | 5.36 | 7.34 | 2.01 | 0.58 | 0.43 |
2.3 Analytical methods
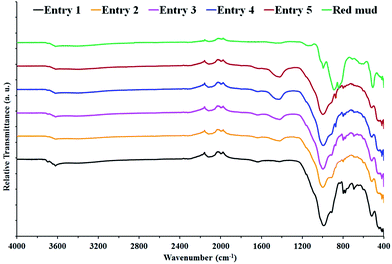
XRD patterns of the sample were determined using a PHILIPS PAN analytical X'Pert X-ray diffractometer with a Cu Kα radiation source, in a 2θ range of 5–90° and at a scanning rate of 2° min−1. The micro-morphology of the sample was investigated using a Scanning Electron Microscope (SEM). Its elemental composition was analyzed using an Energy Dispersive X-ray (EDX), JOEL model, with an Ultimate Resolution JSM-7800F (Japan). The pH measurements were made using a calibrated Orion 2 StarBench top pH meter. The inorganic acid ions of phosphate, sulfate and chloride in the effluent were determined by Ion Chromatography (ICS 1100, American) directly. The FT-IR spectra of the samples were obtained using a PerkinElmer FT-IR Spectrometer Spectrum RX-I. The ratio of sample to KBr was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, and the pallet was prepared at a pressure of 5 tons.
50, and the pallet was prepared at a pressure of 5 tons.
2.4 Experimental set-up
The experimental set up adopted two gas-sealing SCWO reaction vessels developed recently in our laboratory, which were aligned vertically and connected in series. The feedstock (a suspension of sewage sludge and red mud), supercritical water, and oxidant (air) were pumped into the reaction vessels simultaneously from different inlets, while the reaction proceeded. The feedstock inlet was located at the joining flange between the two reaction vessels considering the heating and residence time of the reactants. Supercritical water was pumped in to the bottom of Reaction Vessel 1 (RV1) mainly due to the roles it played as both an energy reservoir and a strong upward carrying fluid. Air was utilized not only as an oxidant but also as a protecting layer for the internal reactor walls, so it was fed from two symmetrical inlets located in RV1 and another two located in RV2. In the reaction zone, various solids (including precipitated inorganic salts, in situ products of neutralization, insoluble or slightly soluble substances in red mud, and sludge ashes) fell down due to natural gravity effects. These dropping solid particles were collected in a compact receptacle fixed to the RV1. Collisions between various particles and flowing fluids further favored the oxidation of pollutants, which was another advantage of the gas-sealing SCWO reaction vessels. More information about the reactor performance and dimensions can be found in other publications.46,47 The flow diagram of this SCWO process is shown in Fig. 1.Prior to each experiment, the feedstock was electrically preheated to the desired inlet temperature. Supercritical water was obtained by heating tap water directly using electricity. Air was also preheated using electricity. For the duration of the experiments, the SCWO reaction vessels continued to be heated electrically to a temperature slightly higher than the test temperature, due to heat-transfer across the vessel walls.
The flowing fluids entered a condenser as they left RV2, in which they were quenched directly by tap water. Then they were further cooled in a heat exchanger. With subsequent depressurization, liquid water and gaseous exhaust were obtained due to phase separation, and both were isolated from the SCWO system without further treatment.
2.5 Experimental procedure
Red mud was added in various proportions to active sewage sludge to make the suspensions used as feedstock. Before feedstock feeding, pre-heated air was constantly pumped into the reaction vessels for at least 10 min so that a gaseous protective layer was formed that acted as a transpiring film similar to the subcritical water film of a conventional transpiring SCWO reactor.48,49 Once conditions reached stationary state, the system was maintained for a few hours to make sufficient processing and neutralization in the reaction vessels. Water samples were taken from the final draining within this stationary operation period. As an experiment was completed, the residue in the solids receptacle was taken out and used directly for analysis.Three groups of experiments were conducted in this work to investigate the impacts of red mud on the SCWO processing of sewage sludge (Table 3). The first group (Entry 1–5) aimed to study the neutralization and catalytic effects of red mud (with different amount of red mud addition) by characterizing both solid residues and drained water. The second (Entry 1–2, 6–9) and third (Entry 1–2, 10–15) group aimed to further study the influences of red mud on the pH control and TOC removal efficiency by the measurements of effluents after SCWO disposal at different operating temperatures and with different residence time, respectively.
| Entry | Feedstock suspension | Air flow rate (L min−1) | Reaction vessels | |||||
|---|---|---|---|---|---|---|---|---|
| Red mud (% w/w) | Sludge (% w/w) | Flow rate (ml min−1) | TOC (mg L−1) | Pressure (MPa) | Temperature (°C) | Residence time (s) | ||
| 1 | 0 | 100% | 20 | 4635.0 | 10 | 26.5 | 400 | 93 |
| 2 | 2% | 98% | 20 | 4542.5 | 10 | 26.5 | 400 | 93 |
| 3 | 4% | 96% | 20 | 4449.9 | 10 | 26.5 | 400 | 93 |
| 4 | 6% | 94% | 20 | 4357.5 | 10 | 26.5 | 400 | 93 |
| 5 | 8% | 92% | 20 | 4264.9 | 10 | 26.5 | 400 | 93 |
| 6 | 0 | 100% | 20 | 4635.0 | 10 | 26.5 | 450 | 93 |
| 7 | 2% | 98% | 20 | 4542.5 | 10 | 26.5 | 450 | 93 |
| 8 | 0 | 100% | 20 | 4635.0 | 10 | 26.5 | 500 | 93 |
| 9 | 2% | 98% | 20 | 4542.5 | 10 | 26.5 | 500 | 93 |
| 10 | 0 | 100% | 15 | 4635.0 | 7.5 | 26.5 | 400 | 124 |
| 11 | 2% | 98% | 15 | 4542.5 | 7.5 | 26.5 | 400 | 124 |
| 12 | 0 | 100% | 12 | 4635.0 | 6 | 26.5 | 400 | 155 |
| 13 | 2% | 98% | 12 | 4542.5 | 6 | 26.5 | 400 | 155 |
| 14 | 0 | 100% | 10 | 4635.0 | 5 | 26.5 | 400 | 186 |
| 15 | 2% | 98% | 10 | 4542.5 | 5 | 26.5 | 400 | 186 |
3. Results and discussion
3.1 Characterization of solid residues
 | ||
| Fig. 3 SEM photography of residues after SCWO treatments (A: red mud; B: Entry 1; C: Entry 2; D: Entry 3; E: Entry 4; F: Entry 5). | ||
Through SEM/EDX analysis, the components of the residue particles were determined and are shown in Tables 4–8. Oxygen, carbon, silicon, aluminum, calcium, and iron were the major elements detected. These results are different from the reports that a pure calcite coating was formed when red mud was used to sequester pure CO2 gas.52 The reason may be due to the different reaction conditions red mud was involved in. In this work, other acidic substances originated from some hetero-atoms were also neutralized simultaneously by CO2 mineralization. The ashes resulting from SCWO processing of pure sludge, as well as from the precipitation of inorganic salts, further complicated the components and microstructure of the residues.
| Elements | % w/w (Spot 1) | % w/w (Spot 2) | % w/w (Spot 3) | % w/w (Spot 4) | % w/w (Spot 5) |
|---|---|---|---|---|---|
| C | 3.85 ± 0.31 | 5.76 ± 0.38 | 6.39 ± 0.47 | 6.88 ± 0.25 | 7.51 ± 0.32 |
| O | 42.33 ± 0.43 | 48.76 ± 0.47 | 33.66 ± 0.54 | 41.01 ± 0.32 | 45.84 ± 0.41 |
| K | 1.41 ± 0.13 | 3.11 ± 0.15 | 3.38 ± 0.19 | 0.42 ± 0.06 | 2.84 ± 0.16 |
| Na | 0.44 ± 0.09 | 0.52 ± 0.08 | — | 0.23 ± 0.05 | — |
| Ca | 9.06 ± 0.13 | 10.54 ± 0.11 | 11.88 ± 0.46 | 12.14 ± 0.18 | 8.46 ± 0.24 |
| Mg | 3.63 ± 0.12 | 1.66 ± 0.09 | 1.41 ± 0.13 | 1.21 ± 0.05 | 1.87 ± 0.12 |
| Al | 16.72 ± 0.17 | 12.19 ± 0.15 | 15.37 ± 0.21 | 12.08 ± 0.06 | 8.77 ± 0.18 |
| Si | 19.86 ± 0.27 | 14.33 ± 0.25 | 25.06 ± 0.31 | 22.28 ± 0.07 | 18.38 ± 0.20 |
| Fe | 2.04 ± 0.47 | 2.31 ± 0.42 | 1.63 ± 0.24 | 3.03 ± 0.36 | 4.27 ± 0.24 |
| P | 0.66 ± 0.08 | 0.54 ± 0.09 | 1.22 ± 0.14 | 0.72 ± 0.14 | 1.59 ± 0.21 |
| S | — | 0.28 ± 0.08 | — | — | 0.47 ± 0.09 |
| Elements | % w/w (Spot 1) | % w/w (Spot 2) | % w/w (Spot 3) | % w/w (Spot 4) | % w/w (Spot 5) |
|---|---|---|---|---|---|
| C | 10.58 ± 0.27 | 8.62 ± 0.45 | 9.87 ± 0.34 | 10.21 ± 0.40 | 13.62 ± 0.51 |
| O | 43.08 ± 0.36 | 43.71 ± 0.48 | 42.84 ± 0.47 | 47.38 ± 0.40 | 45.62 ± 0.37 |
| K | 0.61 ± 0.09 | 1.51 ± 0.11 | 1.46 ± 0.08 | 2.14 ± 0.11 | 0.75 ± 0.08 |
| Na | 0.58 ± 0.07 | 0.96 ± 0.08 | — | — | 0.92 ± 0.14 |
| Ca | 11.78 ± 0.27 | 10.67 ± 0.19 | 12.55 ± 0.24 | 11.13 ± 0.10 | 14.20 ± 0.28 |
| Mg | 0.72 ± 0.09 | 1.41 ± 0.08 | 1.26 ± 0.11 | 1.32 ± 0.07 | 0.87 ± 0.06 |
| Al | 6.72 ± 0.15 | 9.03 ± 0.18 | 6.14 ± 0.14 | 7.42 ± 0.12 | 8.47 ± 0.27 |
| Si | 13.51 ± 0.21 | 12.60 ± 0.21 | 11.47 ± 0.17 | 12.82 ± 0.23 | 11.08 ± 0.15 |
| Fe | 6.76 ± 0.47 | 5.76 ± 0.38 | 7.04 ± 0.44 | 6.08 ± 0.49 | — |
| P | 3.84 ± 0.09 | 2.76 ± 0.12 | 3.71 ± 0.09 | — | 2.07 ± 0.09 |
| S | 1.82 ± 0.06 | 1.45 ± 0.08 | 2.47 ± 0.07 | — | 1.26 ± 0.06 |
| Ti | — | 1.15 ± 0.25 | 1.19 ± 0.06 | 1.50 ± 0.22 | 1.14 ± 0.22 |
| Zr | — | 0.37 ± 0.09 | — | — | — |
| Elements | % w/w (Spot 1) | % w/w (Spot 2) | % w/w (Spot 3) | % w/w (Spot 4) | % w/w (Spot 5) |
|---|---|---|---|---|---|
| C | 16.51 ± 0.47 | 14.77 ± 0.83 | 13.42 ± 0.53 | 19.17 ± 0.67 | 18.89 ± 0.72 |
| O | 38.48 ± 0.57 | 37.61 ± 0.64 | 41.16 ± 0.53 | 35.98 ± 0.39 | 40.10 ± 0.87 |
| K | 0.71 ± 0.11 | 1.32 ± 0.26 | 1.69 ± 0.21 | 0.59 ± 0.07 | 1.44 ± 0.25 |
| Na | — | — | 0.96 ± 0.12 | 0.81 ± 0.05 | — |
| Ca | 12.37 ± 0.21 | 14.24 ± 0.32 | 15.48 ± 0.26 | 12.02 ± 0.43 | 13.55 ± 0.37 |
| Mg | 0.52 ± 0.08 | 0.96 ± 0.12 | 0.74 ± 0.10 | 0.28 ± 0.04 | 1.22 ± 0.11 |
| Al | 2.55 ± 0.13 | 6.59 ± 0.26 | 7.69 ± 0.27 | 9.73 ± 0.08 | 8.42 ± 0.26 |
| Si | 6.33 ± 0.36 | 7.86 ± 0.32 | 7.72 ± 0.35 | 11.51 ± 0.11 | 6.97 ± 0.31 |
| Fe | 9.43 ± 0.37 | 6.44 ± 0.63 | 5.47 ± 0.71 | 6.15 ± 0.39 | 4.88 ± 0.91 |
| P | 5.34 ± 0.17 | 4.91 ± 0.16 | 5.67 ± 0.14 | 3.76 ± 0.06 | 4.53 ± 0.15 |
| S | 3.76 ± 0.14 | 3.41 ± 0.19 | — | — | — |
| Ti | 2.38 ± 0.22 | 1.44 ± 0.23 | — | — | — |
| Zr | — | 0.45 ± 0.13 | — | — | — |
| Ce | 1.62 ± 0.52 | — | — | — | — |
Table 4 revealed the major elemental contents in the residue of Entry 1, which included C (3.85–7.51%), O (33.66–48.76%), Al (8.77–16.72%), Si (14.33–25.06%), Ca (8.46–12.14%) and Fe (1.63–4.27%). With 2, 4, 6, and 8% addition of red mud, the carbon content increased to 8.62–13.62%, 13.42–19.17%, 15.81–22.91%, and 18.76–23.30%, respectively. The calcium content increased to 10.67–14.20%, 12.02–15.48%, 14.67–16.50%, and 14.44–17.10%, respectively. These results demonstrate the absorption of the CO2 produced in situ by red mud, and agree with the incline of calcite amount determined by XRD measurement. The simultaneous increase in the S content from 0.28–0.47% to 1.26–2.47%, 3.41–3.76%, 3.33–4.36%, and 4.47–4.63%, respectively; and P content from 0.54–1.59% to 2.07–3.84%, 3.76–5.67%, 4.73–6.88%, and 4.47–6.82%, respectively, evidenced that other acidic substances produced in situ, were also neutralized inside the SCWO reaction vessels. Furthermore, red mud also answered for recovery of phosphorus during the wastewater treatment process.53 The decline in the aluminum and silicon content was ascribed to the in situ formation of calcite and others of the salts covering up the ash particles. The confirmation of elemental Cl in Tables 7 and 8 was probably due to precipitation of inorganic salts in the SCWO vessels. The increase of Fe, and identification of other elements (including W, Ti, Zr, Ce, and Nd) might be attributed to their introduction in the red mud.
| Elements | % w/w (Spot 1) | % w/w (Spot 2) | % w/w (Spot 3) | % w/w (Spot 4) | % w/w (Spot 5) |
|---|---|---|---|---|---|
| C | 22.91 ± 0.42 | 20.31 ± 0.46 | 18.16 ± 0.36 | 16.73 ± 0.51 | 15.81 ± 0.33 |
| O | 36.50 ± 0.59 | 35.80 ± 0.40 | 40.29 ± 0.41 | 37.24 ± 0.50 | 39.00 ± 0.42 |
| K | 0.53 ± 0.09 | 1.10 ± 0.09 | 0.56 ± 0.08 | 1.41 ± 0.15 | 1.79 ± 0.11 |
| Na | — | 0.20 ± 0.05 | 0.39 ± 0.06 | — | — |
| Ca | 14.67 ± 0.16 | 14.83 ± 0.20 | — | 15.34 ± 0.30 | 16.50 ± 0.23 |
| Mg | 0.41 ± 0.06 | 0.65 ± 0.05 | 0.67 ± 0.06 | 1.21 ± 0.09 | 0.70 ± 0.06 |
| Al | 5.90 ± 0.18 | 4.56 ± 0.10 | 6.54 ± 0.11 | 6.93 ± 0.16 | 7.64 ± 0.14 |
| Si | 5.68 ± 0.15 | 4.41 ± 0.15 | 13.12 ± 0.18 | 6.52 ± 0.25 | 6.41 ± 0.19 |
| Fe | — | 7.61 ± 0.48 | 7.02 ± 0.54 | 8.47 ± 0.79 | 10.53 ± 0.53 |
| P | 6.09 ± 0.16 | 4.80 ± 0.10 | 6.88 ± 0.07 | 4.73 ± 0.12 | — |
| S | 3.60 ± 0.07 | 3.33 ± 0.06 | 4.36 ± 0.06 | — | — |
| Ti | 1.14 ± 0.22 | 1.19 ± 0.30 | 2.01 ± 0.40 | 1.42 ± 0.27 | 1.02 ± 0.25 |
| Zr | — | 1.21 ± 0.33 | — | — | 0.60 ± 0.16 |
| Cl | 0.19 ± 0.06 | — | — | — | — |
| Nd | 0.94 ± 0.64 | — | — | — | — |
| W | 1.44 ± 0.44 | — | — | — | — |
| Elements | % w/w (Spot 1) | % w/w (Spot 2) | % w/w (Spot 3) | % w/w (Spot 4) | % w/w (Spot 5) |
|---|---|---|---|---|---|
| C | 18.76 ± 0.67 | 20.62 ± 0.94 | 19.69 ± 0.64 | 22.89 ± 0.82 | 23.30 ± 0.40 |
| O | 42.75 ± 0.60 | 35.38 ± 0.77 | 36.32 ± 0.65 | 37.01 ± 0.74 | 38.14 ± 0.47 |
| K | 0.73 ± 0.13 | 1.29 ± 0.23 | 1.61 ± 0.17 | 1.47 ± 0.20 | 1.51 ± 0.11 |
| Na | — | — | 0.30 ± 0.10 | — | 0.46 ± 0.06 |
| Ca | 14.44 ± 0.34 | 15.19 ± 0.46 | 16.44 ± 0.28 | 16.24 ± 0.34 | 17.10 ± 0.23 |
| Mg | 0.47 ± 0.09 | 0.53 ± 0.13 | 0.93 ± 0.10 | 1.12 ± 0.12 | 0.89 ± 0.06 |
| Al | 2.01 ± 0.12 | 3.48 ± 0.26 | 2.32 ± 0.25 | 3.31 ± 0.23 | 3.42 ± 0.15 |
| Si | 11.99 ± 0.34 | 4.45 ± 0.36 | 5.13 ± 0.31 | 3.45 ± 0.32 | 2.52 ± 0.20 |
| Fe | — | 7.25 ± 1.25 | 8.53 ± 0.74 | 9.56 ± 0.99 | 6.57 ± 0.52 |
| P | 5.47 ± 0.19 | 4.84 ± 0.19 | 6.82 ± 0.12 | 4.47 ± 0.16 | — |
| S | — | 4.63 ± 0.14 | — | — | 4.47 ± 0.07 |
| Ti | 1.46 ± 0.23 | 1.30 ± 0.25 | 1.42 ± 0.47 | — | 1.10 ± 0.36 |
| Zr | — | — | 0.49 ± 0.16 | 0.48 ± 0.15 | 0.52 ± 0.15 |
| Cl | 0.52 ± 0.09 | 1.04 ± 0.17 | — | — | — |
| Mo | 1.40 ± 0.32 | — | — | — | — |
3.2 Measurements of effluent
With this SCWO process, pH value of the fluid was influenced by the different physical and chemical characteristics it had, as well as the reactions it participated in. In the reaction zone, three types of reaction might occur:
(i) Soluble alkaline components in red mud reacted with acidic substances produced in situ (e.g., CO2).
| OH− + CO2 ↔ CO32− + H2O | (1) |
| 2[Al(OH)4]− + CO2 ↔ 2Al(OH)3 + CO32− + H2O | (2) |
(ii) Some calcium-aluminosilicates/silicates decomposed to free calcium cations, which could react with carbonate/bicarbonate ions to produce calcite.
| Calcium-aluminosilicate/silicate + H2O ↔ Ca2+ + SiO32− | (3) |
| Ca2+ + CO32− + H2O ↔ CaCO3 + H2O | (4) |
| SiO32− + CO2 + H2O ↔ CO32− + H2SiO3 | (5) |
| Ca2+ + 2HCO3− ↔ CaCO3 + CO2 + H2O | (6) |
(iii) CO2 produced in situ reacted with water.
| CO2 + H2O ↔ H2CO3 ↔ H+ + HCO3− ↔ 2H+ + CO32− | (7) |
Compared with other red mud, the red mud used in this study had relatively lower soluble alkaline components represented by the elements Li, Na, and K. As a result, reaction (i) occurred and balanced rapidly. Due to the difficult solubility of aluminum and silicon compounds involved in reaction (ii), the pH of the fluid was mainly determined by the precipitation of calcite and solution of CO2 in the SCWO medium. As presented by the EDX measurement, some newly formed inorganic salts that contained sulfur, phosphorus and chloride elements were deposited due to the non-polar characteristic of the supercritical fluid. Combined with the decline of these elemental acid ions in the effluents with the red mud amount added (Table 9), the neutralization of in situ formed acidic substances was indicated. And it could be concluded that at the outlet of RV2, the fluid mainly contain soluble inorganic salts (SIS), remaining organic substances (RS), as well as gases of CO2, N2 and O2.
| Contents (mg L−1) | Entry 1 | Entry 2 | Entry 3 | Entry 4 | Entry 5 |
|---|---|---|---|---|---|
| PO43− | 129.54 ± 3.81 | 68.22 ± 4.69 | 46.78 ± 3.75 | 21.12 ± 3.92 | 4.55 ± 2.68 |
| SO42− | 161.57 ± 4.78 | 57.12 ± 3.83 | 42.92 ± 3.67 | 36.16 ± 3.91 | 18.28 ± 2.87 |
| Cl− | 73.48 ± 2.89 | 49.39 ± 1.95 | 38.67 ± 3.82 | 27.81 ± 2.78 | 25.35 ± 2.75 |
With cooling, the fluid tended to reach a nearly ambient temperature. As a result, a new balance of SIS–RS–CO2–H2O, at a certain pH value, was established. Due to the subsequent depressurization operation, the over-dissolved CO2 at high pressures were released and the pH was determined by the newly formed balance of SIS–RS–CO2–H2O at ambient temperature and pressure. This was the pH buffering mechanism of red mud during the SCWO processing of sewage sludge.
With 2% of red mud addition, a rise of pH was observed at each operating temperature of 400, 450 and 500 °C (Fig. 7A). And the pH incline resulting from the temperature increase (pure sewage sludge) did not alleviate the neutralization effect of red mud. However, due to the reduction of RS with operating temperature increase, SIS–CO2–H2O system was more likely than the SIS–RS–CO2–H2O to control the pH of the drained water. Compared with the processing of pure sewage sludge, the effluent was neutralized by the red mud although different residence time was applied (Fig. 7B). However, this neutralization effect was weakened (the pH decreased from 7.67 to 7.32, 7.05 and 6.68) as the residence time increased. The reason may be resulted from the decrease of SIS in the fluid due to the precipitation of various solids on the surfaces of red mud particles. As a result, the pH of the drained water was mainly controlled by the system of RS–CO2–H2O while a long residence time was adopted.
 | ||
| Fig. 8 TC and TOC/TC values in the effluents (A); influences of red mud addition on the TOC removal rates for the sewage sludge disposal (B). | ||
In this work, the CE can be defined as
 | (8) |
As shown in Fig. 9A, a rise of TOC removal rate was observed with 2% red mud addition at each operating temperature of 400, 450 and 500 °C. And the improvement of TOC removal efficiency (from 92.21% to 95.26% and 97.84%) caused by the temperature increase did not interfere with the catalytic effect of red mud. With the CE values of 40.56%, 56.12% and 84.72% observed at temperature of 400, 450 and 500 °C, respectively, the enhancement of catalytic effect resulting from the temperature increase was confirmed. This can be explained by the decreased maximum catalytic capacity (1 − R1), as well as other factors leading to the rapid pollutants oxidation reaction (increase of R2).
 | ||
| Fig. 9 Catalytic effects of red mud during the SCWO disposal of sewage sludge. (A: At different operating temperatures; B: with different residence time at 400 °C). | ||
During the SCWO processing of sewage sludge with red mud additive, the catalytic effect probably comes from two aspects: active components of red mud particles and fine grains formed in situ. At the primary period, the direct contact between red mud particles and other reactants (e.g., pollutants, oxygen gas) in the reaction zone, active components such as oxides of iron,59 cerium,60 manganese,61 titanium,35 might promote oxidation reactions effectively. The elements lithium, sodium, and potassium also play important roles for the catalytic oxidation of sludge pollutants.62–65 At the subsequent period, calcite and other inorganic salts were formed in situ and surrounded the ash particles with the neutralization of acidic substances. These fine grains contact organic pollutants and oxygen gas, especially later in the oxidation reaction, causing a catalytic effect that result in further oxidation. In the experiments with different residence time, both of the TOC removal rate and CE value increased monotonously as the residence time increased. And a TOC removal rate of 97.12% was obtained in entry 15, with the corresponding CE value of 63.03% (Fig. 9B). These results strongly demonstrate that the catalytic effect of red mud can be highly enhanced by the constantly in situ forming fine grains of calcite, tricalcium aluminate, perovskite, hematite, and other inorganic salts. By introducing Ca(NO3)2 into a SCWO reactor, the CaCO3 formed in situ was reported to act as a catalyst for the decomposition of acrylonitrile.66 In a near-critical water medium, calcite can enhance the oxidation rate of alcohols,67 and many metals contained in sewage sludge such as Pb, Ni, Zn, Cu and Cr can be effectively mineralized.68
4. Conclusions
Red mud was used in the process of SCWO disposal of sewage sludge, not only as an acid neutralizer but also as an oxidation catalyst. With addition of 2, 4, 6, and 8% of red mud, the TC value of the solid residue increased from 9.16 mg g−1 (for pure sludge processing), to 14.88, 17.91, 20.11, and 26.14 mg g−1. This mainly resulted from the net increase of inorganic carbon content attributed to the capture and absorption of CO2 produced in situ. The pH of the drained water was correspondingly increased from 5.16 to a stable range of 7.67–7.82 due to addition of the red mud. This was mainly determined by the balance of the SIS–RS–CO2–H2O system at ambient conditions after cooling and depressurization. However, the system of SIS–CO2–H2O was more likely to control the pH of the effluent as the operating temperature increased due to the reduction of RS. With the increase of residence time, the neutralization effect of red mud was weakened. The reason was probably attributed to the decrease of SIS in the fluid, and the pH was mainly controlled by the system of RS–CO2–H2O.An increase in the TOC removal rate was observed with increasing red mud content, and a rate in excess of 99.3% could be achieved by the addition of 8% red mud, although at a relatively low temperature of 400 °C. Combined with the rise of TOC removal rate after 2% red mud addition confirmed in the experiments with different operating temperatures and that with different residence time, the catalytic effect of red mud was strongly evidenced. It was probably attributable to the active components red mud contained at the primary period, and to the fine-grained calcite that formed in situ, along with tricalcium aluminate, perovskite, hematite, and other inorganic salts at the subsequent period. In the practical application, both of the neutralization effect and the catalytic effect of red mud could be controlled by the change of detailed conditions (e.g., addition amount, operating temperature, residence time).
After the processes of neutralization, oxidation, and other chemical or physical processes, such as mixing of ashes and precipitation of inorganic salts, relatively stable solid residues were obtained in this work. These could easily be handled with conventional methods, or recycled for a remarkable range of industrial applications including such as building materials, water treatment materials, ceramics, and silicate cements.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (grant number: 41203047), the Frontier and Application Foundation Research Program of Chongqing (grant number: cstc2015jcyjA20008), the Key Laboratory for Solid Waste Management and Environment Safety Open Fund (grant number: SWMES 2013-06), 100-Talent Program of Chinese Academia of Sciences (Y33Z050M10) and the two Key Technology R&D Programs of Chongqing (grant number: cstc2012gg-sfgc20001 and cstc2011ggC20014).References
- J. A. Melero, R. S. Vázquez, I. A. Vasiliadou, F. M. Castillejo, L. F. Bautista, J. Iglesias, G. Morales and R. Molina, Municipal sewage sludge to biodiesel by simultaneous extraction and conversion of lipids, Energy Convers. Manage., 2015, 103, 111–118 CrossRef CAS.
- L. Qian, S. Wang, D. Xu, Y. Guo, X. Tang and L. Wang, Treatment of sewage sludge in supercritical water and evaluation of the combined process of supercritical water gasification and oxidation, Bioresour. Technol., 2015, 176, 218–224 CrossRef CAS PubMed.
- M. Samolada and A. Zabaniotou, Comparative assessment of municipal sewage sludge incineration, gasification and pyrolysis for a sustainable sludge-to-energy management in Greece, Waste Manag., 2014, 34, 411–420 CrossRef CAS PubMed.
- C. Valderrama, R. Granados, J. L. Cortina, C. M. Gasol, M. Guillem and A. Josa, Comparative LCA of sewage sludge valorisation as both fuel and raw, J. Cleaner Prod., 2013, 51, 205–213 CrossRef CAS.
- S. Donatello and C. R. Cheeseman, Recycling and recovery routes for incinerated sewage sludge ash (ISSA): a review, Waste Manag., 2013, 33, 2328–2340 CrossRef CAS PubMed.
- A. K. Kleczkowska, K. Środa, M. K. Golachowska, T. Musiał and K. Wolski, Mechanisms and kinetics of granulated sewage sludge combustion, Waste Manag., 2015, 46, 459–471 CrossRef PubMed.
- W. Deng, J. Yan, X. Li, F. Wang, Y. Chi and S. Lu, Emission characteristics of dioxins, furans and polycyclic aromatic hydrocarbons during fluidized-bed combustion of sewage sludge, J. Environ. Sci., 2009, 21, 1747–1752 CrossRef CAS.
- L. Batistella, V. Silva, R. C. Suzin, E. Virmond, C. A. Althoff, R. F. P. M. Moreira and H. J. José, Gaseous emissions from sewage sludge combustion in a moving bed combustor, Waste Manag., 2015, 46, 430–439 CrossRef CAS PubMed.
- G. Mininni, A. Sbrilli, E. Guerriero and M. Rotatori, Dioxins and furans formation in pilot incineration tests of sewage sludge spiked with organic chlorine, Chemosphere, 2004, 54, 1337–1350 CrossRef CAS PubMed.
- Z. Xiao, X. Yuan, H. Li, L. Jiang, L. Leng, X. Chen, G. Zeng, F. Li and L. Cao, Chemical speciation, mobility and phyto-accessibility of heavy metals in fly ash and slag from combustion of pelletized municipal sewage sludge, Sci. Total Environ., 2015, 536, 774–783 CrossRef CAS PubMed.
- X. Niu, L. Shen, H. Gu, S. Jiang and J. Xiao, Characteristics of hematite and fly ash during chemical looping combustion of sewage sludge, Chem. Eng. J., 2015, 268, 236–244 CrossRef CAS.
- M. J. Cocero, E. Alonso, M. T. Sanz and F. Fdz-Polanco, Supercritical water oxidation process under energetically self-sufficient operation, J. Supercrit. Fluids, 2002, 24, 37–46 CrossRef CAS.
- M. D. Bermejo and M. J. Cocero, Destruction of an industrial wastewater by supercritical water oxidation in a transpiring wall reactor, J. Hazard. Mater., 2006, 137, 965–971 CrossRef CAS PubMed.
- M. D. Bermejo, P. Cabeza, J. P. S. Queiroz, C. Jiménez and M. J. Cocero, Analysis of the scale up of a transpiring wall reactor with a hydrothermal flame as a heat source for the supercritical water oxidation, J. Supercrit. Fluids, 2011, 56, 21–32 CrossRef CAS.
- D. D. Macdonald and L. B. Kriksunov, Probing the chemical and electrochemical properties of SCWO systems, Electrochim. Acta, 2001, 47, 775–790 CrossRef CAS.
- B. Cui, F. Cui, G. Jing, S. Xu, W. Hu and S. Liu, Oxidation of oily sludge in supercritical water, J. Hazard. Mater., 2009, 165, 511–517 CrossRef CAS PubMed.
- D. Zou, Y. Chi, J. Dong, C. Fu, F. Wang and M. Ni, Supercritical water oxidation of tannery sludge: Stabilization of chromium and destruction of organics, Chemosphere, 2013, 93, 1413–1418 CrossRef CAS PubMed.
- A. Miller, R. Espanani, A. Junker, D. Hendry, N. Wilkinson, D. Bollinger, J. M. Abelleira-Pereira, M. A. Deshusses, E. Inniss and W. Jacoby, Supercritical water oxidation of a model fecal sludge without the use of a co-fuel, Chemosphere, 2015, 141, 189–196 CrossRef CAS PubMed.
- L. Qian, S. Wang, D. Xu, Y. Guo, X. Tang and L. Wang, Treatment of municipal sewage sludge in supercritical water: a review, Water Res., 2016, 89, 118–131 CrossRef CAS PubMed.
- P. Kritzer and E. Dinjus, An assessment of supercritical water oxidation (SCWO) existing problems, possible solutions and new reactor concepts, Chem. Eng. J., 2001, 83, 207–214 CrossRef CAS.
- M. Svanstrom, M. Froling, M. Modell, W. A. Peters and J. Tester, Environmental assessment of supercritical water oxidation of sewage sludge, Resour., Conserv. Recycl., 2004, 41, 321–338 CrossRef.
- P. A. Marrone, Supercritical water oxidation-current status of full-scale commercial activity for waste destruction, J. Supercrit. Fluids, 2013, 79, 283–288 CrossRef CAS.
- M. Hodes, P. A. Marrone, G. T. Hong, K. A. Smith and J. W. Tester, Salt precipitation and scale control in supercritical water oxidation-part A: fundamentals and research, J. Supercrit. Fluids, 2004, 29, 265–288 CrossRef CAS.
- P. A. Marrone, M. Hodes, K. A. Smith and J. W. Tester, Salt precipitation and scale control in supercritical water oxidation-part B: commercial/full-scale applications, J. Supercrit. Fluids, 2004, 29, 289–312 CrossRef CAS.
- M. H. Delville, P. Botella, T. Jaszay and J. P. Frayret, Electrochemical study of corrosion in aqueous high pressure, high temperature media and measurements of materials corrosion rates: applications to the hydrothermal treatments of organic wastes by SCWO, J. Supercrit. Fluids, 2003, 26, 169–179 CrossRef CAS.
- P. Kritzer, Corrosion in high-temperature and supercritical water and aqueous solutions: a review, J. Supercrit. Fluids, 2004, 29, 1–29 CrossRef CAS.
- R. M. Serikawa, T. Usui, T. Nishimura, H. Sato, S. Hamada and H. Sekino, Hydrothermal flames in supercritical water oxidation: investigation in a pilot scale continuous reactor, Fuel, 2002, 81, 1147–1159 CrossRef CAS.
- V. Marulanda and G. Bolaños, Supercritical water oxidation of a heavily PCB-contaminated mineral transformer oil: laboratory scale data and economic assessment, J. Supercrit. Fluids, 2010, 54, 258–265 CrossRef CAS.
- D. H. Xu, S. Z. Wang, Y. M. Gong, Y. Guo, X. Y. Tang and H. H. Ma, A novel concept reactor design for preventing salt deposition in supercritical water, Chem. Eng. Res. Des., 2010, 88, 1515–1522 CrossRef CAS.
- M. Schubert, J. Aubert, J. B. Müller and F. Vogel, Continuous salt precipitation and separation from supercritical water. Part 3: Interesting effects in processing type 2 salt mixtures, J. Supercrit. Fluids, 2012, 61, 44–54 CrossRef CAS.
- D. Xu, C. Huang, S. Wang, G. Lin and Y. Guo, Salt deposition problems in supercritical water oxidation, Chem. Eng. J., 2015, 279, 1010–1022 CrossRef CAS.
- C. Sun, R. Hui, W. Qu and S. Yick, Progress in corrosion resistant materials for supercritical water reactors, Corros. Sci., 2009, 51, 2508–2523 CrossRef CAS.
- P. A. Morrone and G. T. Hong, Corrosion control methods in supercritical water oxidation and gasification processes, J. Supercrit. Fluids, 2009, 51, 83–103 CrossRef.
- M. Krajnc and J. Levec, The role of catalyst in supercritical water oxidation of acetic acid, Appl. Catal., B, 1997, 13, 93–103 CrossRef.
- C. B. Maugans and A. Akgerman, Catalytic wet oxidation of phenol in a trickle bed reactor over a Pt/TiO2 catalyst, Water Res., 2003, 37, 319–328 CrossRef CAS PubMed.
- Y. H. Shin, H. S. Lee, Y. H. Lee, J. Kim, J. D. Kim and Y. W. Lee, Synergetic effect of copper-plating wastewater as a catalyst for the destruction of acrylonitrile wastewater in supercritical water oxidation, J. Hazard. Mater., 2009, 167, 824–829 CrossRef CAS PubMed.
- X. Dong, Z. Gan, X. Lu, W. Jin, Y. Yu and M. Zhang, Study on catalytic and non-catalytic supercritical water oxidation of p-nitrophenol wastewater, Chem. Eng. J., 2015, 277, 30–39 CrossRef CAS.
- S. Samal, A. K. Ray and A. Bandopadhyay, Proposal for resources, utilization and processes of red mud in India-a review, Int. J. Miner. Process., 2013, 118, 43–55 CrossRef CAS.
- W. Liu, X. Chen, W. Li, Y. Yu and K. Yan, Environmental assessment, management and utilization of red mud in China, J. Cleaner Prod., 2014, 84, 606–610 CrossRef CAS.
- G. Power, M. Gräfe and C. Klauber, Bauxite residue issues: I. Current management, disposal and storage practices, Hydrometallurgy, 2011, 108, 33–45 CrossRef CAS.
- M. Johnston, M. W. Clark, P. McMahon and N. Ward, Alkalinity conversion of bauxite refinery residues by neutralization, J. Hazard. Mater., 2010, 182, 710–715 CrossRef CAS PubMed.
- V. S. Yadav, M. Prasad, J. Khan, S. S. Amritphale, M. Singh and C. B. Raju, Sequestration of carbon dioxide (CO2) using red mud, J. Hazard. Mater., 2010, 176, 1044–1050 CrossRef CAS PubMed.
- L. J. Kirwan, A. Hartshorn, J. B. McMonagle, L. Fleming and D. Funnell, Chemistry of bauxite residue neutralization and aspects to implementation, Int. J. Miner. Process., 2013, 119, 40–50 CrossRef CAS.
- S. Sushil and V. S. Batra, Catalytic applications of red mud, an aluminium industry waste: a review, Appl. Catal., B, 2008, 81, 64–77 CrossRef CAS.
- S. Wang, H. M. Ang and M. O. Tadé, Novel applications of red mud as coagulant, adsorbent and catalyst for environmentally benign processes, Chemosphere, 2008, 72, 1621–1635 CrossRef CAS PubMed.
- Z. Chen, G. Wang, Z. A. Mirza, S. Yang and Y. Xu, Study of transpiring fluid dynamics in supercritical water oxidation using a transparent reactor, J. Supercrit. Fluids, 2014, 88, 117–125 CrossRef CAS.
- Z. Chen, G. Wang, F. Yin, H. Chen and Y. Xu, A new system design for supercritical water oxidation, Chem. Eng. J., 2015, 269, 343–351 CrossRef CAS.
- M. D. Bermejo, F. Fdez-Polanco and M. J. Cocero, Experimental study of the operational parameters of a transpiring wall reactor for supercritical water oxidation, J. Supercrit. Fluids, 2006, 39, 70–79 CrossRef CAS.
- D. Xu, S. Wang, C. Huang, X. Tang and Y. Guo, Transpiring wall reactor in supercritical water oxidation, Chem. Eng. Res. Des., 2014, 92(11), 2626–2639 CrossRef CAS.
- B. I. Whittington and C. M. Cardile, The chemistry of tricalcium aluminate hexahydrate relating to the Bayer industry, Int. J. Miner. Process., 1996, 48, 21–38 CrossRef CAS.
- C. Liao, L. Zeng and K. Shih, Quantitative X-ray Diffraction (QXRD) analysis for revealing thermal transformations of red mud, Chemosphere, 2015, 131, 171–177 CrossRef CAS PubMed.
- R. C. Sahu, R. K. Patel and B. C. Ray, Neutralization of red mud using CO2 sequestration cycle, J. Hazard. Mater., 2010, 179, 28–34 CrossRef CAS PubMed.
- W. Huang, S. Wang, Z. Zhu, L. Li, X. Yao, V. Rudolph and F. Haghseresht, Phosphate removal from wastewater using red mud, J. Hazard. Mater., 2008, 158, 35–42 CrossRef CAS PubMed.
- H. Nath, P. Sahoo and A. Sahoo, Characterization of red mud treated under high temperature fluidization, Powder Technol., 2015, 269, 233–239 CrossRef CAS.
- R. Senthil Kumar and P. Rajkumar, Characterization of minerals in air dust particles in the state of Tamilnadu, India through FTIR, XRD and SEM analyses, Infrared Phys. Technol., 2014, 67, 30–41 CAS.
- L. Qian, S. Wang, D. Xu, Y. Guo, X. Tang and L. Wang, Treatment of sewage sludge in supercritical water and evaluation of the combined process of supercritical water gasification and oxidation, Bioresour. Technol., 2015, 176, 218–224 CrossRef CAS PubMed.
- Y. Gong, S. Wang, H. Xu, Y. Guo and X. Tang, Partial oxidation of landfill leachate in supercritical water: optimization by response surface methodology, Waste Manag., 2015, 43, 343–352 CrossRef CAS PubMed.
- D. Zou, Y. Chi, C. Fu, J. Dong, F. Wang and M. Ni, Co-destruction of organic pollutants in municipal solid waste leachate and dioxins in fly ash under supercritical water using H2O2 as oxidant, J. Hazard. Mater., 2013, 248–249, 177–184 CrossRef CAS PubMed.
- C. T. Wang and R. J. Willey, Oxidation of methanol over iron oxide based aerogels in supercritical CO2, J. Non-Cryst. Solids, 1998, 225, 173–177 CrossRef CAS.
- A. Trovarelli, C. de Leitenburg, M. Boaro and G. Dolcetti, The utilization of ceria in industrial catalysis, Catal. Today, 1999, 50, 353–367 CrossRef CAS.
- X. Dong, Z. Gan, X. Lu, W. Jin, Y. Yu and M. Zhang, Study on catalytic and non-catalytic supercritical water oxidation of p-nitrophenol wastewater, Chem. Eng. J., 2015, 277, 30–39 CrossRef CAS.
- K. S. Lin, H. P. Wang and Y. W. Yang, Supercritical water oxidation of 2-chlorophenol effected by Li+ and CuO/zeolites, Chemosphere, 1999, 39(9), 1385–1396 CrossRef CAS.
- G. Lee, T. Nunoura, Y. Matsumura and K. Yamamoto, Comparison of the effects of the addition of NaOH on the decomposition of 2-chlorophenol and phenol in supercritical water and under supercritical water oxidation conditions, J. Supercrit. Fluids, 2002, 24, 239–250 CrossRef CAS.
- Z. Fang, S. K. Xu, R. L. Smith Jr., K. Arai and J. A. Kozinski, Destruction of deca-chlorobiphenyl in supercritical water under oxidizing conditions with and without Na2CO3, J. Supercrit. Fluids, 2005, 33, 247–258 CrossRef CAS.
- Z. Sun, F. Takahashi, Y. Odaka, K. Fukushi, Y. Oshima and K. Yamamoto, Effects of potassium alkalis and sodium alkalis on the dechlorination of o-chlorophenol in supercritical water, Chemosphere, 2007, 66, 151–157 CrossRef CAS PubMed.
- Y. H. Shin, H. Lee, B. Veriansyah, J. Kim, D. S. Kim, H. W. Lee, Y. S. Youn and Y. W. Lee, Simultaneous carbon capture and nitrogen removal during supercritical water oxidation, J. Supercrit. Fluids, 2012, 72, 120–124 CrossRef CAS.
- G. J. Suppes, S. Roy and J. Ruckman, Calcium carbonate catalysis of alcohol oxidation in near-critical water, AIChE J., 2001, 47, 2102–2110 CrossRef CAS.
- E. Yabalak and A. M. Giair, Subcritical and supercritical fluid extraction of heavy metals from sand and sewage sludge, J. Serb. Chem. Soc., 2013, 78, 1013–1022 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |