Ferroelectric-like hysteresis effect observed in carbon quantum dots sandwiched between PMMA and PEDOT:PSS hybrid film
Abstract
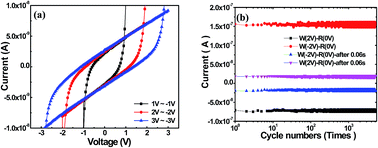
The ferroelectric-like hysteretic I–V effect was observed in the multilayer film of PMMA/carbon quantum dot (CD)/PEDOT:PSS using ITO and Al metal as the electrodes. The multilayer film exhibited two different resistance states and larger hysteresis window under the electric field compared to the film without CD addition. The influence of additional CD layers on the short circuit current (Isc) and open circuit voltage (Voc) values is related to the interfacial polarization between PMMA and PEDOT:PSS layers. The introduction of a CD layer intensifies interfacial polarization for two dielectric layers, resulting in the larger hysteresis window. An interfacial polarization field E′ increases with the external electric field increasing and reaches a maximum value at the threshold voltage Voc of ±15 V. The switch reliability of the device is operated over 5 × 103 times in a succession of periodic pulses without any performance degradation.

 Please wait while we load your content...
Please wait while we load your content...