Preparation and engineering of oriented 2D covalent organic framework thin films†
Abstract
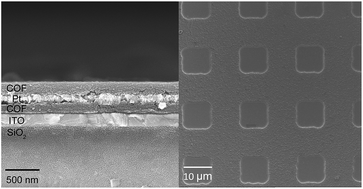
We report herein the preparation and engineering of oriented thin films of a two-dimensional (2D) covalent organic framework (COF). The 2D COF, constructed by condensing p-phenylenediamine (DAB) with 1,3,5-triformylphlorogluciol (TFP), was solvothermally grown and formed oriented thin films on several substrates. Two strategies were developed to fabricate well-defined metal/COF multi-layered structures and to pattern the obtained COF thin films. The first strategy is based on alternating physical deposition of metal and chemical deposition of COF, and the second is realized via photolithography and reactive ion etching techniques.

 Please wait while we load your content...
Please wait while we load your content...