Development of a macroporous-spherical polyanionic compound (TiO)2P2O7 as a novel anode material for sodium ion batteries
Shuming Zhang ,
Yu Liu
,
Yu Liu *,
Na Zhang,
Kuan Zhao,
Jianhua Yang and
Shiyang He
*,
Na Zhang,
Kuan Zhao,
Jianhua Yang and
Shiyang He
Power Energy Storage Technology and Application Center, Shanghai Institute of Ceramics, Chinese Academy of Sciences, Shanghai 200050, P. R. China. E-mail: yuliu@mail.sic.ac.cn; Fax: +86 21 69987795; Tel: +86 21 69987795
First published on 25th May 2016
Abstract
The phosphate-based polyanionic compound with the formula (TiO)2P2O7 was synthesized by a simple precipitation method with a soft template as a novel anode material for sodium ion batteries for the first time. The polyanionic compound (TiO)2P2O7 with a macroporous-spherical structure exhibited an initial specific capacity of 293.0 mA h g−1 at 0.1C in the voltage range of 0.01–2.5 V vs. Na+/Na. The reversible capacity remained at 200.9 mA h g−1 after 100 cycles. It is attractive that the macroporous-spherical (TiO)2P2O7 delivered an extremely stable cycling performance at a high charge rate of 2.5C. A high capacity retention of 85.1% was obtained after 1100 cycles with an initial specific capacity of 112.7 mA h g−1. The ex situ XRD and XPS analysis indicates that the excellent cycle stability and high rate capability are attributed to the intrinsic 3D framework structure and well-connected macroporous-spherical morphology of (TiO)2P2O7. The macroporous-spherical (TiO)2P2O7 is a promising anode for sodium ion batteries.
Introduction
Sodium ion batteries (SIBs) are promising candidates for energy storage technology due to their low cost and the relative abundance of sodium.1–3 Due to the larger radius of the sodium ion (1.02 Å for Na+ vs. 0.76 Å for Li+),4 it is difficult to have an appropriate host to possess an open structure and thermal stability to accommodate enough sodium ion sites and to ensure rapid and reversible insertion/extraction of sodium ions.5,6Ti-based oxides such as TiO2 are attractive candidates for SIB anodes owing to the merits such as nontoxicity, low-cost, intrinsic safety and easy preparation.7,8 However, TiO2 based anodes shows poor cycling stability and unsatisfactory specific capacity. Modification of the morphology as nanoparticles,9 nanotubes10 and porous structures,11,12 as well as the design of carbon based composites13,14 is an effective approach to improve the electrochemical performances of TiO2.
On the other hand, phosphate based compounds have been identified as a potential anode candidate for sodium ion storage due to their highly covalent 3D framework structure that generates large interstitial spaces for sodium ion diffusion without lattice perturbation during the intercalation/de-intercalation process.15,16 In fact, there are a series of polyanionic compounds based on P2O74−, such as TiP2O7,17 SnP2O7,18 Fe2P2O7,19 (TiO)2P2O7 (ref. 20) and (VO)2P2O7.21 As a typical P2O74−-based polyanionic material, TiP2O7, which has a cubic superstructure cell like NaCl-type structure, replacing Na+ site by TiO6 octahedra and Cl− site by P2O7 double-tetrahedra, has been previously investigated for the potential application in catalysis and batteries.22–24 However, the rate performance of pristine TiP2O7 was greatly hindered by sluggish kinetics of electron transfer.22,25
In this work, a Ti-phosphate based polyanionic compound (TiO)2P2O7 was developed as a novel anode for sodium ion batteries. The (TiO)2P2O7 contains the P2O7 double-tetrahedra and TiO6 octahedra to form 3D framework structure. It was found that the (TiO)2P2O7 with macroporous spherical morphology delivered a outstanding rate performance and a high cycle stability.
Experimental
Preparation of (TiO)2P2O7 microparticles
The macroporous-spherical (TiO)2P2O7 microparticles, here denoted as P-TOPO, were prepared by a simple precipitation method with a soft template. Titanous sulfate (Ti(SO4)2), ammonium dihydrogen phosphate (NH4H2PO4) and polyethylene glycol (PEG) were chosen as the raw materials. Ti(SO4)2 and NH4H2PO4 with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were dissolved in 100 mL deionized water under ultrasonic treatment to form a clear solution separately. A certain amount of PEG and H2O2 (30%) was added into Ti(SO4)2 solution. After being stirred at room temperature for 10 min, NH4H2PO4 solution was rapidly transferred into Ti(SO4)2 solution with violent magnetic stirring. After being stirred at a mild temperature of 80 °C for 5 h, the milky white suspension formed in the solution was subsequently filtered and washed with deionized water and then dried in an oven at 100 °C for 12 h. The as-prepared dried powers were ground and annealed at 900 °C for 5 h. For the comparison, the spherical (TiO)2P2O7 microparticles (denoted as S-TOPO) were synthesized under the same conditions without the addition of H2O2.
1 were dissolved in 100 mL deionized water under ultrasonic treatment to form a clear solution separately. A certain amount of PEG and H2O2 (30%) was added into Ti(SO4)2 solution. After being stirred at room temperature for 10 min, NH4H2PO4 solution was rapidly transferred into Ti(SO4)2 solution with violent magnetic stirring. After being stirred at a mild temperature of 80 °C for 5 h, the milky white suspension formed in the solution was subsequently filtered and washed with deionized water and then dried in an oven at 100 °C for 12 h. The as-prepared dried powers were ground and annealed at 900 °C for 5 h. For the comparison, the spherical (TiO)2P2O7 microparticles (denoted as S-TOPO) were synthesized under the same conditions without the addition of H2O2.
Structural and morphological characterization
The crystal structures of the synthesized materials were analyzed by X-ray diffraction (XRD) using a Rigaku Ultima IV diffractometer with Cu-Kα radiation (λ = 1.5418 Å) at a scanning rate of 2° min−1. The X-ray photoelectron spectroscopy analysis (XPS) was carried out on ESCALAB 250. The morphological characteristics of the (TiO)2P2O7 microparticles were observed by scanning electron microscopy (SEM, JSM-6510, JEOL, Japan) and transmission electron microscopy (TEM, JEM-2100F, JEOL, Japan).Electrochemical characterization
The given amount of (TiO)2P2O7 active material, acetylene black and polyvinylidene fluoride (PVDF) with a weight ratio of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 were mixed by the aid of 1-methyl-2-pyrrolidinone (NMP) to form the slurry. The (TiO)2P2O7 electrodes were prepared by spreading the homogeneous slurry onto Cu foil and then dried at 100 °C in a vacuum oven for 12 h. The mass of active material could be estimated as 1–2 mg on the Cu foil with a diameter of 12 mm. The CR2032 coin-type cells were assembled with the (TiO)2P2O7 electrode, sodium metal as the counter electrode and microporous polypropylene membrane (Celgard 2400) as the separator in a glove box by limiting the moisture and oxygen below 0.1 ppm. The electrolyte was 1 M NaClO4 in ethylene carbonate (EC)/dimethyl carbonate (DMC) (1
10 were mixed by the aid of 1-methyl-2-pyrrolidinone (NMP) to form the slurry. The (TiO)2P2O7 electrodes were prepared by spreading the homogeneous slurry onto Cu foil and then dried at 100 °C in a vacuum oven for 12 h. The mass of active material could be estimated as 1–2 mg on the Cu foil with a diameter of 12 mm. The CR2032 coin-type cells were assembled with the (TiO)2P2O7 electrode, sodium metal as the counter electrode and microporous polypropylene membrane (Celgard 2400) as the separator in a glove box by limiting the moisture and oxygen below 0.1 ppm. The electrolyte was 1 M NaClO4 in ethylene carbonate (EC)/dimethyl carbonate (DMC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume). Cyclic voltammetry (CV) curves with a voltage range of 0.01–3.0 V were obtained at a scanning rate of 0.1 mV s−1 by using a CHI 604E electrochemical workstation (Shanghai CH Instruments Inc. China). Galvanostatic charge/discharge test was carried out in a potential range of 0.01–2.5 V (vs. Na+/Na) by using a LAND CT2001A battery tester (Wuhan LANHE Inc. China) under various current densities. Electrochemical impedance spectroscopy (EIS) was measured by using an Autolab4.9 potentiostat (PGSTAT302N) in the frequency range from 100 kHz to 0.01 Hz.
1 in volume). Cyclic voltammetry (CV) curves with a voltage range of 0.01–3.0 V were obtained at a scanning rate of 0.1 mV s−1 by using a CHI 604E electrochemical workstation (Shanghai CH Instruments Inc. China). Galvanostatic charge/discharge test was carried out in a potential range of 0.01–2.5 V (vs. Na+/Na) by using a LAND CT2001A battery tester (Wuhan LANHE Inc. China) under various current densities. Electrochemical impedance spectroscopy (EIS) was measured by using an Autolab4.9 potentiostat (PGSTAT302N) in the frequency range from 100 kHz to 0.01 Hz.
Results and discussion
Structural and morphological characterization
The XRD patterns of the as-synthesized P-TOPO ((TiO)2P2O7 under the synthesis condition with H2O2) and S-TOPO ((TiO)2P2O7 under the synthesis condition without H2O2) are shown in Fig. 1. The diffraction peaks are mainly indexed as (TiO)2P2O7 (JCPDS no. 39-0207).20 A small amount of TiP2O7 impurity phase was detected in the raw material. It was found that the crystallinity of the P-TOPO sample is weaker than that of the S-TOPO. This could be ascribed to the porous morphology of P-TOPO by the effect of H2O2 as the foaming agent.26 | ||
| Fig. 1 XRD patterns of the dense spherical (TiO)2P2O7 (S-TOPO) (a) and the macroporous spherical (TiO)2P2O7 (P-TOPO) (b). | ||
Fig. 2 shows the SEM images of the spherical (TiO)2P2O7 particles. As can be seen in Fig. 2a, the S-TOPO displays a spherical and dense structure with a particle size ranging from 5 to 7 μm. The enlarged image given in Fig. 2b further indicates that the S-TOPO has a strawberry like morphology. The SEM images of the P-TOPO in Fig. 2c and d indicate that the addition of H2O2 is in favor of obtaining a loose and porous structure. TEM in Fig. 3a further confirms that the S-TOPO has a relatively dense and compact structure. By contrast, it is obvious that the P-TOPO has a macroporous structure containing a large number of holes, as shown in Fig. 3b.
The pore size distribution of the (TiO)2P2O7 materials is further investigated via nitrogen adsorption–desorption tests and the Brunauer–Emmett–Teller (BET) adsorption isotherms. In Fig. 4, the isotherms of P-TOPO show a typical type IV curve with a hysteresis loop in the range of 0.5–0.9P/P0, which is indicative of a macroporous feature of the synthesized P-TOPO sample. The BET specific surface area and pore volume of the P-TOPO sample are determined to be 114 m2 g−1 and 0.7 cm3 g−1, respectively. The Barrett–Joyner–Halenda (BJH) pore size distribution shown in the inset of Fig. 4 indicates that the P-TOPO exhibits macropores in the pore size ranged from 60 to 140 nm. In contrast, the S-TOPO sample has no type IV curve, which is in well agreement with the morphology of S-TOPO in Fig. 3a. The BET specific surface area of the S-TOPO sample is determined to be 0.014 m2 g−1 without pore volume. As expected, the highly porous characteristics of P-TOPO result in large specific surface area, which is a benefit for the electrochemical performance of (TiO)2P2O7 materials.
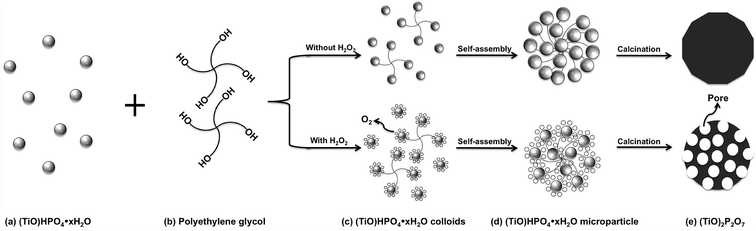
The possible mechanism for the formation of the P-TOPO is proposed and illustrated in Fig. 5. NH4H2PO4 reacted with Ti(SO4)2 to form titanyl hydrogen phosphate hydrate (TiO)HPO4·xH2O, as given in Fig. 5a. It further combined with the PEG molecular chains. In the same time under high temperature H2O2 decomposed to be O2, which was absorbed on the surface of (TiO)HPO4·xH2O, as shown in Fig. 5c. The (TiO)HPO4·xH2O nanoparticles with the adsorbed oxygen preferred to aggregate and self-assembled to form the spherical microparticles, as given in Fig. 5d. The holes through the (TiO)2P2O7 microparticles were created to give the macroporous structure after the calcinations, as shown in Fig. 5e.
Mechanism analysis
The cyclic voltammetry (CV) profile of the P-TOPO shown in Fig. 6a indicates that two oxidation/reduction peaks appeared at 1.9/1.8 V (Ti4+/Ti3+ redox couples) and 0.52/0.32 V (Ti3+/Ti2+ redox couples) at the first cycle.16,27 In the following cycles the peak at the 0.32 V for the Ti3+/Ti2+ reduction had a small shifting towards negative potential. Fig. 6b further shows the charge and discharge curves of the (TiO)2P2O7 electrode at 0.1C in the voltage range of 0.01–2.5 V. The electrochemical behaviors of the P-TOPO are similar to those of the reported TiO2.8 The cell had a slope potential platform along with a reversible capacity of around 200.9 mA h g−1 during the 100 cycles. An obvious irreversible capacity lose was observed at the first cycle. This could be attributed to the irreversible sodium ion loss for the phase transform and the inevitable side reaction with electrolyte to form a SEI film.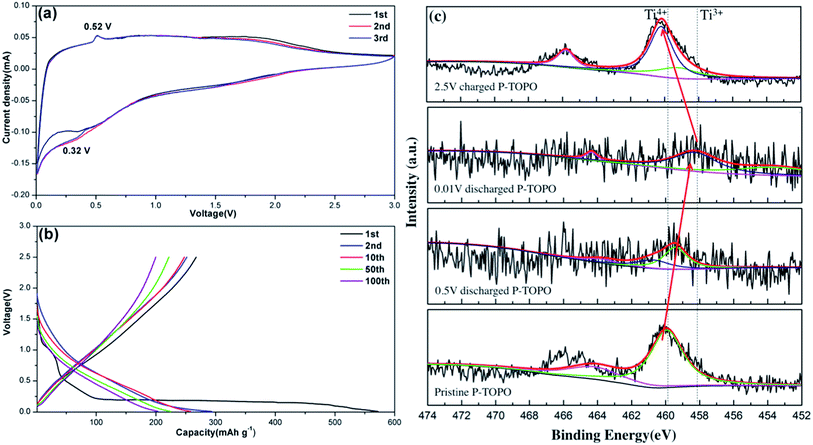 | ||
| Fig. 6 (a) CV curves of P-TOPO at a scan rate of 0.1 mV s−1; (b) charge–discharge curves of P-TOPO at 0.1C (1C = 386 mA h g−1); (c) ex situ XPS studies of P-TOPO electrodes. | ||
The ex situ XPS studies in Fig. 6c clarify the oxidation states of the titanium under sodium ion insertion and extraction for the P-TOPO. A peak at the binding energy of 459.7 eV was related to Ti4+ for the starting material. It shifted to 458.3 eV at the fully discharged state and returned to the original value upon the fully re-charged process. It indicates that the valence state of the titanium in TOPO is Ti2+/Ti3+ and Ti3+/Ti4+ for the discharge and charge process, respectively. The above results show that the as-prepared P-TOPO may have the intercalation capability of three Na-ions per formula unit.
Ex situ XRD was performed for the P-TOPO at the first discharge–charge cycle and the following second discharge stage, as shown in Fig. 7. The new reflection peaks were observed at 10.9°, 17.0°, 30.5°, 33.2° and 37.0° compared with the starting (TiO)2P2O7 as the first discharge voltage reached at around 0.3 V. The peaks continually enhanced upon the discharge stage up to 0.01 V. The appeared peaks could correspond to the formation of the Na3(TiO)2P2O7 under a possible reaction of (TiO)2P2O7 + 3Na+ + 3e = Na3(TiO)2P2O7. The reflection peaks exist during the first charge and the following second discharge. The result is in the good agreement of the charge and discharge profiles shown in Fig. 6b.
 | ||
| Fig. 7 Ex situ XRD patterns of P-TOPO during the first discharge–charge and the following second discharge. | ||
Electrochemical characterization
The cycling performance of the S-TOPO electrode and the P-TOPO electrode at 0.1C is shown in Fig. 8a. After 100 cycles, a reversible capacity of 152.3 mA h g−1 was obtained for the S-TOPO electrode. The P-TOPO electrode shows a much higher cycling stability over the S-TOPO electrode. A high capacity of 200.9 mA h g−1 was obtained for the P-TOPO electrode upon 100 cycles. The charge rate of the P-TOPO electrode is superior to that of the S-TOPO, as shown in Fig. 8b. A high capacity of 80.8 mA h g−1 was observed for the P-TOPO at 5C. The excellent rate capability could be due to the macroporous structure of the P-TOPO, which is a benefit for the improvement of the reaction area.Fig. 8c shows the long durability upon cycles of the P-TOPO electrode at 2.5C. After 1100 cycles the reversible capacity of the P-TOPO electrode maintains at 95.3 mA h g−1, which is twice as much as that of the S-TOPO electrode (about 43 mA h g−1). The excellent cycling stability and rate performance are ascribed to the 3D framework structure and well-connected porous morphology of the P-TOPO. The SEM images of P-TOPO electrodes before cycle and after the 30th and 60th cycles are shown in Fig. 9. It is found from Fig. 9a that visible spherical morphology of P-TOPO can be clearly seen on the surface of the electrode before cycle. After the 30th and 60th cycles (Fig. 9b and c), respectively, the spherical morphology is almost remained without disintegration. This indicates a highly structural stability of the P-TOPO active hosts during the repeated sodium ion insertion and extraction.
The electrochemical impedance spectra (EIS) spectra of the S-TOPO and P-TOPO electrodes were measured in fully charged states after different charge/discharge cycles at 0.1C and shown in Fig. 10a and b. The Nyquist plots can be fitted and interpreted well based on the equivalent electric circuit,28 as shown in Fig. 10c. The large semicircle is related to the charge transfer resistance (Rct) and constant phase element (CPE2). Another indistinct small high-frequency semicircle stemming from Rf and CPE1 is ascribed to the SEI layer, which is similar to that in LIBs. Rs and Zw represent ohmic resistance including the electrolyte, separator and electrical contacts, as well as the Warburg impedance of Na-ion diffusion into active materials, respectively. The fitted Rs, Rf and Rct are listed in Table 1. It can be clearly observed that the Rct decreases with the increase of cycle numbers, which can be ascribed to the electrode activation phenomenon.29 The Rct of the P-TOPO electrode is obviously smaller than that of S-TOPO electrode, indicating that the well-connected porous morphology is highly useful for the electrochemical reaction for the (TiO)2P2O7 based electrode.
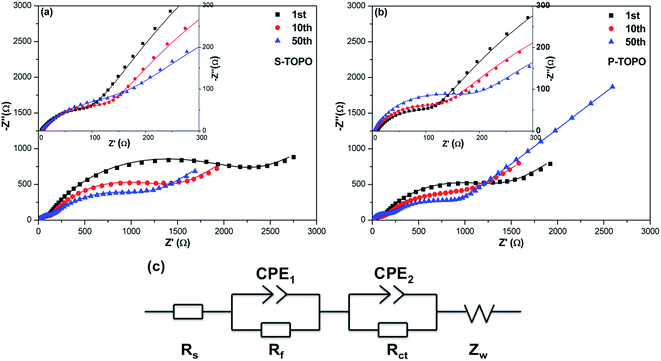 | ||
| Fig. 10 Nyquist plots measured for S-TOPO (a) and P-TOPO (b) in fully charged states after different cycling numbers from 100 kHz to 0.01 Hz; (c) the equivalent circuit model. | ||
| Cycles | S-TOPO | P-TOPO | ||||
|---|---|---|---|---|---|---|
| Rs (ohm) | Rf (ohm) | Rct (ohm) | Rs (ohm) | Rf (ohm) | Rct (ohm) | |
| 1st | 4.45 | 87.8 | 1930 | 8.98 | 93.3 | 1080 |
| 10th | 5.03 | 113 | 1130 | 4.72 | 119 | 745 |
| 50th | 5 | 153 | 897 | 3.09 | 175 | 551 |
Conclusions
In this work, the phosphate-based polyanionic compound, (TiO)2P2O7, has been successfully synthesized through a simple precipitation method with a soft template. It was found that the electrochemical performance of the (TiO)2P2O7 spherical particles with a porous morphology is obviously superior to that of the (TiO)2P2O7 with a dense structure. The macroporous-spherical (TiO)2P2O7 delivered a reversible capacity of 200.9 mA h g−1 at a current density of 0.1C after 100 cycles. A reversible capacity of 95.9 mA h g−1 was maintained after 1100 cycles at a high charge rate of 2.5C. The macroporous-spherical (TiO)2P2O7 is a promising anode candidate for sodium ion batteries.Acknowledgements
This work was financially supported by the Shanghai Science and Technology Commission (STCSM) Project No. 14DZ1201500.Notes and references
- S.-W. Kim, D.-H. Seo, X. Ma, G. Ceder and K. Kang, Adv. Energy Mater., 2012, 2, 710–721 CrossRef CAS.
- H. Pan, Y.-S. Hu and L. Chen, Energy Environ. Sci., 2013, 6, 2338 CAS.
- M. D. Slater, D. Kim, E. Lee and C. S. Johnson, Adv. Funct. Mater., 2013, 23, 947–958 CrossRef CAS.
- N. Yabuuchi, K. Kubota, M. Dahbi and S. Komaba, Chem. Rev., 2014, 114, 11636–11682 CrossRef CAS PubMed.
- V. Palomares, P. Serras, I. Villaluenga, K. B. Hueso, J. Carretero-González and T. Rojo, Energy Environ. Sci., 2012, 5, 5884 CAS.
- J. Liu, J.-G. Zhang, Z. Yang, J. P. Lemmon, C. Imhoff, G. L. Graff, L. Li, J. Hu, C. Wang, J. Xiao, G. Xia, V. V. Viswanathan, S. Baskaran, V. Sprenkle, X. Li, Y. Shao and B. Schwenzer, Adv. Funct. Mater., 2013, 23, 929–946 CrossRef CAS.
- J. Xu, Y. Wang, Z. Li and W. F. Zhang, J. Power Sources, 2008, 175, 903–908 CrossRef CAS.
- D. Yan, C. Yu, Y. Bai, W. Zhang, T. Chen, B. Hu, Z. Sun and L. Pan, Chem. Commun., 2015, 51, 8261–8264 RSC.
- L. Wu, D. Buchholz, D. Bresser, L. Gomes Chagas and S. Passerini, J. Power Sources, 2014, 251, 379–385 CrossRef CAS.
- X. Yang, C. Wang, Y. Yang, Y. Zhang, X. Jia, J. Chen and X. Ji, J. Mater. Chem. A, 2015, 3, 8800–8807 CAS.
- H.-E. Wang, H. Cheng, C. Liu, X. Chen, Q. Jiang, Z. Lu, Y. Y. Li, C. Y. Chung, W. Zhang, J. A. Zapien, L. Martinu and I. Bello, J. Power Sources, 2011, 196, 6394–6399 CrossRef CAS.
- H. E. Wang, Z. G. Lu, L. J. Xi, R. G. Ma, C. D. Wang, J. A. Zapien and I. Bello, ACS Appl. Mater. Interfaces, 2012, 4, 1608–1613 CAS.
- F. Yang, Z. Zhang, Y. Han, K. Du, Y. Lai and J. Li, Electrochim. Acta, 2015, 178, 871–876 CrossRef CAS.
- Y. Ge, H. Jiang, J. Zhu, Y. Lu, C. Chen, Y. Hu, Y. Qiu and X. Zhang, Electrochim. Acta, 2015, 157, 142–148 CrossRef CAS.
- K. Saravanan, C. W. Mason, A. Rudola, K. H. Wong and P. Balaya, Adv. Energy Mater., 2013, 3, 444–450 CrossRef CAS.
- P. Senguttuvan, G. Rousse, M. E. Arroyo y de Dompablo, H. Vezin, J. M. Tarascon and M. R. Palacin, J. Am. Chem. Soc., 2013, 135, 3897–3903 CrossRef CAS PubMed.
- H. Wang, K. Huang, Y. Zeng, S. Yang and L. Chen, Electrochim. Acta, 2007, 52, 3280–3285 CrossRef CAS.
- Y. Li and J. Li, J. Phys. Chem. C, 2008, 112, 14216–14219 CAS.
- Q. Shi, L. Zhang, M. E. Schlesinger, J. Boerio-Goates and B. F. Woodfield, J. Chem. Thermodyn. Thermochem., 2013, 61, 51–57 CrossRef CAS.
- C. E. Bamberger and G. M. Begun, J. Less-Common Met., 1987, 134, 201–206 CrossRef CAS.
- D. W. Aldous, R. J. Goff, J. P. Attfield and P. Lightfoot, Inorg. Chem., 2007, 46, 1277–1282 CrossRef CAS PubMed.
- Y. Sun, L. Gai, Y. Zhou, X. Zuo, J. Zhou and H. Jiang, CrystEngComm, 2014, 16, 10681–10691 RSC.
- A. K. Rai, J. Gim, J. Song, V. Mathew, L. T. Anh and J. Kim, Electrochim. Acta, 2012, 75, 247–253 CrossRef CAS.
- P. Senguttuvan, G. Rousse, J. Oró-Solé, J. M. Tarascon and M. R. Palacín, J. Mater. Chem. A, 2013, 1, 15284–15291 CAS.
- A. K. Padhi, J. Electrochem. Soc., 1997, 144, 2581 CrossRef CAS.
- Y. Fang, L. Xiao, J. Qian, X. Ai, H. Yang and Y. Cao, Nano Lett., 2014, 14, 3539–3543 CrossRef CAS PubMed.
- Y. Kim, K.-s. Park, S.-h. Song, J. Han and J. B. Goodenough, J. Electrochem. Soc., 2009, 156, A703 CrossRef CAS.
- K. Chang and W. Chen, ACS Nano, 2011, 5, 4720–4728 CrossRef CAS PubMed.
- J. Xiao, X. Wang, X.-Q. Yang, S. Xun, G. Liu, P. K. Koech, J. Liu and J. P. Lemmon, Adv. Funct. Mater., 2011, 21, 2840–2846 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |