In2O3-functionalized MoO3 heterostructure nanobelts with improved gas-sensing performance†
Abstract
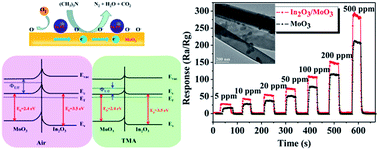
A novel heterostructure of In2O3 nanoparticle-functionalized MoO3 nanobelts was synthesized via a simple solution method. The phase purity, morphology and structure of the as-prepared In2O3-functionalized MoO3 heterostructure nanobelts were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and X-ray photoelectron spectroscopy (XPS). To demonstrate the potential applications of such In2O3/MoO3 composites, the as-prepared products were used to fabricate a gas sensor that was then investigated for gas-sensing performances. Results of the test showed that the response of In2O3 nanoparticle-functionalized MoO3 nanobelts against 10 ppm trimethylamine (TMA) is up to 31.69 at the working temperature of 260 °C, which is higher than that of bare MoO3 nanobelts. Moreover, the In2O3-functionalized MoO3 heterostructure sensor also exhibits excellent selectivity and rapid response and recovery speed. Such behaviors are attributed to the combination of In2O3 nanoparticles and uniformly decorated MoO3 nanobelts endowing a fascinating sensing performance for a novel sensing material, which may provide a new strategy to enhance the performance of sensing materials in the application of gas sensors.

 Please wait while we load your content...
Please wait while we load your content...