Electrochemical reduction of hydrogen peroxide by nanostructured hematite modified electrodes†
Chia-Ting Chang and
Chia-Yu Lin*
National Cheng Kung University, No. 1, University Road, Tainan City 70101, Taiwan. E-mail: cyl44@mail.ncku.edu.tw
First published on 4th July 2016
Abstract
In this study, various nanostructured hematites (α-Fe2O3), including nanorods (α-Fe2O3NR), nanoparticles (α-Fe2O3Np), and nanosheets (α-Fe2O3NS), were synthesized and their electrocatalytic properties towards the reduction of H2O2 were investigated. All nanostructured α-Fe2O3 hematites were synthesized using chemical bath deposition (CBD) under mild conditions, followed by thermal treatment at 500 °C. The nanostructure was controlled simply by adjusting the composition of precursor solution and reaction duration for the CBD process. It was found that iron phosphate (FePO4) was deposited in situ onto the surface of these nanostructured α-Fe2O3 hematites during the electrochemical pretreatment in the phosphate electrolyte, and both FePO4 and α-Fe2O3 showed activity in catalysing the electrochemical reduction of H2O2. In addition, the interaction/compatibility between deposited FePO4 and α-Fe2O3 has a decisive effect on the overall electrocatalytic activity of the resultant electrodes; FePO4 only showed a synergetic effect on the overall electrocatalytic activity with α-Fe2O3NR and α-Fe2O3NS. The rate constant is highest for the electro-reduction of H2O2 on FePO4 modified α-Fe2O3NR (α-Fe2O3NR|FePO4), but FePO4 modified α-Fe2O3NS (α-Fe2O3NS|FePO4) showed the best overall electrocatalytic activity due to its relatively higher surface area. Furthermore, dissolved oxygen showed negligible interference on the activity of α-Fe2O3NR|FePO4 and α-Fe2O3NS|FePO4, which makes them promising sensing materials in oxidase-based electrochemical sensors.
Introduction
The development of a highly sensitive and reliable hydrogen peroxide (H2O2) electrochemical sensor is of great importance not only because H2O2 has been identified as a chemical threat to the environment and a major factor causing some diseases,1 but also it a frequent intermediate involved in many important oxidase-catalyzed chemical processes, such as glucose oxidation catalysed by glucose oxidase (GOD) (eqn (1) and (2)):2| Glucose + GOD–FAD ⇄ gluconolactone + GOD–FADH2 | (1) |
| O2 + GOD–FADH2 → H2O2 + GOD–FAD | (2) |
H2O2 is an electroactive species that can be oxidized or reduced electrochemically, and therefore, the electrochemical detection of chemicals involved in oxidase-catalyzed chemical processes can be achieved by detection of H2O2. Some oxidase-based electrochemical sensors that utilize the anodic current from the electrooxidation of H2O2 as the output signal have been developed, but these kinds of sensors often suffer interference from some common electro-oxidizable species, such as ascorbic acid and uric acid existing in biological samples.3 As a result, to minimize the interference and enhance the selectivity of oxidase-based electrochemical sensors, the development of oxidase-based electrochemical sensors that use the cathodic current from the electroreduction of H2O2 as the output signal is preferred. However, in this case, dissolved oxygen, required to re-oxidize the oxidase (e.g., eqn (2)), becomes a potential interfering species as oxygen can also be reduced electrochemically. Therefore, an electrocatalyst that can selectively catalyse the reduction of H2O2 against the reduction of O2 is highly required for constructing oxidase-based electrochemical sensors that operate in a cathodic regime.
Many materials have been explored as active species to catalyze the electrochemical reduction of H2O2, including prussian blue,4 iron oxides,5 silver,6 manganese oxides,7 copper oxides,8 etc. Among them, iron oxides have received much attention not only because they are robust, earth abundant, and can be easily synthesized in a cheap way, but also it exhibited peroxidase-like activity.9 Nevertheless, the mechanism for the electrocatalysis of H2O2 by iron oxides is still not well-understood. For example, in a previous report,10 iron oxide nanorods, including β-FeOOH, α-Fe2O3, γ-Fe2O3, were found to be active for the electrochemical reduction of H2O2 in non-phosphate buffer, but only α-Fe2O3 was found to be active in the phosphate buffer. The interaction/compatibility between the surface modifier (iron phosphate) and the iron oxide matrix played an important role in determining the overall activity of the iron oxide based material. On the other hand, iron oxides of various nanostructures, such as nanorods,5f nanoparticles,5b–d,f,11 and nanotubes,5e have been synthesized, and these nanostructured iron oxides have been shown to exhibit enhanced apparent electrocatalytic activity compared with the bulk counter-parts. Crystallinity, crystal size, structure, and exposed surface facets, have been shown to have decisive effects on the overall activity of these nanostructured iron oxides.11c,12 Nevertheless, most nanostructured iron oxides were synthesized in powder form, and rarely directly deposited onto an electrode surface, which would not only cause irreproducibility due to the uncontrollable aggregation of these nano-sized iron oxides, but also complicate the following electrode preparation process.
In this work, we report the direct growth of hematite (α-Fe2O3) with different nanostructures, including nanorods, nanosheets, and nanoparticles, onto a fluorine-doped tin oxide coated glass substrate (FTO) using chemical bath deposition under mild conditions with follow-up thermal treatment. The effects of nanostructure and the interplay of FePO4 with different nanostructured α-Fe2O3 on the overall electrocatalytic properties towards the reduction of H2O2 were thoroughly investigated. It was found that synergetic effects of FePO4 with α-Fe2O3 greatly enhanced the overall electrocatalytic activity, in terms of overpotential and catalytic current, compared with FePO4 or α-Fe2O3 alone, but this effect occurs only for α-Fe2O3 nanorods and nanosheets. In addition, the detection of H2O2 by Fe2O3NR|FePO4 and Fe2O3NS|FePO4 is insensitive to the dissolved oxygen, which allows their application towards electrochemical detection of key biomolecules involving in the oxidase-catalyzed chemical processes.
Experimental section
General consideration
The starting materials for the synthetic part of the work were purchased from commercial suppliers and are of the highest available purity for the analytical work. Fluorine-doped tin oxide (FTO) coated glass (sheet resistance 7 ohm sq−1, TEC GlassTM 7) substrates (1.0 × 3.0 cm2) were cleaned with an ammonia–hydrogen peroxide–deionized water mixture (volume ratio: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) at 70 °C for 30 min, after which the FTO substrates were dried at room temperature under a nitrogen purge. A hydrogen peroxide stock solution (0.5 M) was prepared before each experiment by direct dilution of hydrogen peroxide (H2O2, 30 wt%) with electrolyte solutions, of different pHs, either containing (i) sodium phosphate (0.1 M) and sodium sulfate (0.1 M), or (ii) sodium sulfate (0.1 M). Deionized water (DIW) was used throughout the work.
5) at 70 °C for 30 min, after which the FTO substrates were dried at room temperature under a nitrogen purge. A hydrogen peroxide stock solution (0.5 M) was prepared before each experiment by direct dilution of hydrogen peroxide (H2O2, 30 wt%) with electrolyte solutions, of different pHs, either containing (i) sodium phosphate (0.1 M) and sodium sulfate (0.1 M), or (ii) sodium sulfate (0.1 M). Deionized water (DIW) was used throughout the work.
Preparation of the FTO|α-Fe2O3NR, FTO|α-Fe2O3NS and FTO|α-Fe2O3NP electrodes
The FTO|α-Fe2O3NR electrode was prepared by first growing akaganeite nanorods (NR) onto the FTO substrate using chemical bath deposition (CBD) in an aqueous solution containing 1.0 M urea and 0.15 M iron chloride at 90 °C for 4 h, followed by thermal conversion of akaganeite NRs to α-Fe2O3NR at 500 °C for 1 h. The α-Fe2O3 nanosheets (α-Fe2O3NS) were grown onto the FTO substrate, designated as FTO|α-Fe2O3NS, by CBD in an aqueous solution containing 0.75 M urea and 0.15 M iron nitrate at 90 °C for 4 h and follow-up thermal treatment at 500 °C for 1 h. The α-Fe2O3 nanoparticles (α-Fe2O3NP) were grown onto the FTO substrate, designated as FTO|α-Fe2O3NP, by CBD in an aqueous solution containing 2.0 M urea and 0.15 M iron nitrate at 90 °C for 24 h and follow-up thermal treatment at 500 °C for 1 h. The exposed area of the FTO substrate for growing nanostructured hematite was kept at 2.0 cm2.Physical characterization
The surface morphology of the electrodes was characterized using scanning electron microscopy (SEM, Hitachi SU-8010). X-Ray diffraction (XRD) analyses were carried out using an Ultima IV (Rigaku Co., Japan) X-ray diffractometer. The surface composition of the films was verified by X-ray photoelectron spectroscopy (XPS, PHI 5000 VersaProbe system, ULVAC-PHI, Chigasaki, Japan), using a microfocused (100 μm, 25 W) Al X-ray beam, with a photoelectron take off angle of 45°. The Ar+ ion source for XPS (FIG-5CE) was controlled using a floating voltage of 0.2 kV. The binding energies obtained in the XPS analyses were corrected for specimen charging, by referencing the C 1s peak to 285.0 eV.Electrochemical characterization
Electrochemical characterizations of the electrocatalytic properties of the nanostructured hematite modified electrodes were performed with a CHI 660 electrochemical workstation (CH Instruments, Inc., USA) at room temperature and all potentials are reported against Ag/AgCl (saturated KCl). A conventional three-electrode electrochemical cell was employed with nanostructure hematite modified electrodes (exposed area of ∼1.0 cm2) as the working electrode, Pt foil (exposed area 4.0 cm2) as counter electrode, and Ag/AgCl as a reference electrode. Prior to experiments, all hematite modified electrodes were pretreated either in (i) phosphate buffer solution (PBS, pH 7) containing sodium phosphate (0.1 M) and sodium sulfate (0.1 M), or in (ii) sodium sulfate (0.1 M, pH 7), using cyclic voltammetry (CV) at a scan rate of 50 mV s−1 in the potential window between −0.7 V and +0.4 V (vs. Ag/AgCl) until the CV curves became stabilised. The sensitivities of all the hematite modified electrodes determined by CV were the slopes of the curves of cathodic peak current density vs. H2O2 concentration. A suitable operating potential in the limiting current plateau region for the amperometric detection of H2O2 was determined using linear sweep voltammetry (LSV) at a scan rate of 0.1 mV s−1 in PBS (pH 6) containing 0 mM and 4.95 mM H2O2. After obtaining the operating potential, which is −0.3 V vs. Ag/AgCl, the amperometric detection of H2O2 was carried out in PBS (pH 6) under constant magnetic stirring. The current density responses to the change in H2O2 concentration were collected, and the calibration curve for the detection of H2O2 was then constructed. All the electrochemical measurements were repeated at least three times.Results and discussion
Synthesis of the nanostructured hematite electrodes
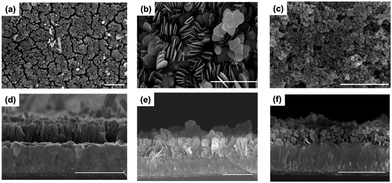
All nanostructured hematites (α-Fe2O3) were directly grown on to FTO by chemical bath deposition under mild conditions and follow-up thermal treatment at 500 °C for 1 h (see ESI† for details). The XRD analyses (Fig. S1†) show that all deposited materials were converted into hematite after thermal treatment. As revealed in the SEM images, shown in Fig. 1, the nanostructure of α-Fe2O3 can be controlled by tuning the composition of the bath solution and the reaction times. For example, a nanorod array (α-Fe2O3NR) can be grown in a FeCl3–urea bath solution system, whereas nanosheets (α-Fe2O3NS) and nanoparticles (α-Fe2O3NP) can be grown in a Fe(NO3)3–urea bath solution system. The adsorption of anions (Cl−, CO32−, NO3−) to specific crystal faces and their relative concentrations influence the preferential growth direction of crystals, and therefore, different nanostructures are created. The detailed growth mechanism will be submitted elsewhere soon. The relative effective surface area for these nanostructured α-Fe2O3 hematites was determined by measuring double-layer capacitance using cyclic voltammetry,13 and the results (Fig. S2†) reveal that the relative effective surface area (α-Fe2O3NR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) α-Fe2O3NS
α-Fe2O3NS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) α-Fe2O3NP) is 1.00
α-Fe2O3NP) is 1.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.58
1.58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.74.
1.74.
Electrochemical characterization
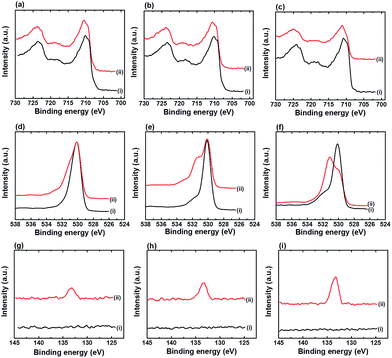
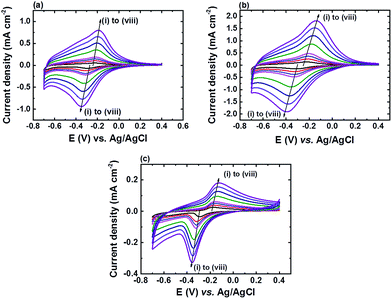
α-Fe2O3 is studied in this work as we found that only α-Fe2O3 is compatible with in situ deposited iron phosphate (FePO4) in phosphate buffer.10 As the formation of FePO4 on an α-Fe2O3 surface during the electrochemical detection of H2O2 in phosphate buffer solution (PBS) is inevitable, and to ensure the surface of all the nanostructured α-Fe2O3 modified electrodes are fully covered with FePO4, all the nanostructured α-Fe2O3 modified electrodes were pre-treated in 0.1 M PBS solution (pH 7) using cyclic voltammetry (CV) from +0.4 to −0.7 V vs. Ag/AgCl at a scan rate of 50 mV s−1 until the redox peaks of FePO4 were saturated. Fig. 2 shows the XPS spectra of Fe 2p, O 1s, and P 2p for all three nanostructured α-Fe2O3 electrodes before and after CV pre-treatment. A positive shift (from ∼710.9 to 711.3 eV) in binding energy (BE) of the Fe 2p peak after the pre-treatment was noticed for all the electrodes (Fig. 2a–c). In addition, the appearance of an additional shoulder in the O 1s spectra at a BE of 531.5 eV (Fig. 2d–f) along with a peak in the P 2p spectra at a BE of 133.2 eV (Fig. 2g–i) after the pre-treatment was also observed for all the electrodes.Fig. 3 shows the CV curves of all the pre-treated nanostructured α-Fe2O3 modified electrodes in PBS (pH 7) at different scan rates (v) and the corresponding plots of the peak current density (Jp) vs. v are shown in Fig. S3.† It can be found that the all the pre-treated electrodes exhibited reversible redox peaks which are characteristic of FePO4,14 and the relationship between Jp with v is linear (see Fig. S3†), which suggests that the deposited species strongly absorbed onto the electrode surface after the pre-treatment. The above observations (Fig. 2 and 3) suggest that FePO4 formed during the pre-treatment process. In addition, the slopes of the plot Jpc vs. v for α-Fe2O3NR|FePO4, α-Fe2O3NS|FePO4, and α-Fe2O3NP|FePO4 are found to be −8.38, −18.45, and −2.72, respectively, and the ratio of the relative amount of the deposited FePO4 on the pre-treated α-Fe2O3NR, α-Fe2O3NS, and α-Fe2O3NP can be deduced according to eqn (3), which is 1.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.20
2.20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.32. Nevertheless, from the XPS analyses, it was found that the elemental ratios of P/Fe for α-Fe2O3NR|FePO4, α-Fe2O3NS|FePO4, and α-Fe2O3NP|FePO4 are 0.26, 0.47, and 0.85, respectively. These findings suggest that the surface of α-Fe2O3NP prefers the adsorption of phosphate ions over the deposition of FePO4.
0.32. Nevertheless, from the XPS analyses, it was found that the elemental ratios of P/Fe for α-Fe2O3NR|FePO4, α-Fe2O3NS|FePO4, and α-Fe2O3NP|FePO4 are 0.26, 0.47, and 0.85, respectively. These findings suggest that the surface of α-Fe2O3NP prefers the adsorption of phosphate ions over the deposition of FePO4.
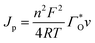 | (3) |
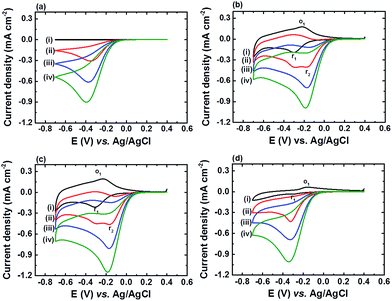
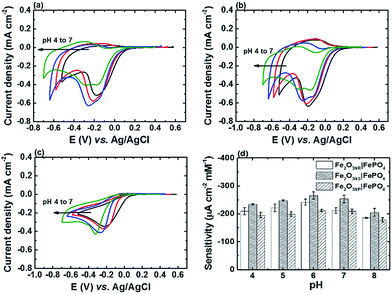
Fig. 4 shows the CV curves of FTO, α-Fe2O3NR|FePO4, α-Fe2O3NS|FePO4, and α-Fe2O3NP|FePO4 at a scan rate of 20 mV s−1 in PBS solution (pH 7) containing H2O2 of various concentrations. The cathodic peak current densities (Jpc) and corresponding peak potentials (Epc) of all the electrodes in the presence of 4.95 mM H2O2 are also summarized in Table 1. It can be found that all FePO4 modified α-Fe2O3 electrodes exhibited better electrocatalytic activity, in terms of Jpc and Epc, than the FTO substrate. In addition, Jpc at E = −0.30 V vs. Ag/AgCl (r1) increased and Jpa at E = −0.22 V vs. Ag/AgCl (o1) decreased upon the addition of H2O2 for α-Fe2O3NR|FePO4, α-Fe2O3NS|FePO4, and α-Fe2O3NP|FePO4, which indicates that the electrochemical process at peak r1 involves an electrocatalytic EC′ mechanism, and the deposited FePO4 is the active species responsible for the electrochemical process. In addition, an additional cathodic peak (r2) at E = −0.18 V vs. Ag/AgCl appeared upon the addition of H2O2 to α-Fe2O3NR|FePO4 and α-Fe2O3NS|FePO4, and as this peak is more sensitive to H2O2 than peak r1, peak r2 outpaced peak r1 at a H2O2 concentration higher than 3.31 mM. Fig. 5a–c show the CV curves of α-Fe2O3NR|FePO4, α-Fe2O3NS|FePO4, and α-Fe2O3NP|FePO4, respectively, in PBS containing 1.66 mM H2O2 at various pHs ranging from 4 to 7. It can be found that the Epc for peak r1 for all the α-Fe2O3 modified electrodes shifted to the more negative side as the solution pH was increased, which reflects the redox behavior of FePO4, whereas the Epc of peak r2 (only for α-Fe2O3NR|FePO4 and α-Fe2O3NS|FePO4) was insensitive to the change in solution pH. Fig. 5d shows the sensitivities, i.e., the slope of the curve of Jpc vs. H2O2 concentration, of α-Fe2O3NR|FePO4, α-Fe2O3NS|FePO4, and α-Fe2O3NP|FePO4, towards the electrochemical reduction of H2O2 at various solution pHs. It can be found that all the electrodes showed their best sensitivity at pH 6, and α-Fe2O3NS|FePO4 exhibited the highest sensitivity among the three α-Fe2O3 modified electrodes. Fig. S4† shows the chronoamperometric response of α-Fe2O3NS|FePO4, at an applied potential of −0.3 V vs. Ag/AgCl, after successive addition of the H2O2 solution of various concentrations into deaerated 0.1 M PBS (pH 6). It can be found that the current response increased linearly with the increase in H2O2 concentration. The sensitivity, i.e., the slope of the calibration curve (shown in the inset of Fig. S4†) was found to be 225.0 ± 19.9 μA cm−2 mM−1. Besides, the sensor response reached 95% of the steady-state value within 10 s upon the addition of H2O2. Furthermore, a limit of detection (signal to noise ratio = 3) of 3.4 ± 0.5 μM can be achieved.
| Sample | ||||
|---|---|---|---|---|
| FTO | α-Fe2O3NR|FePO4 | α-Fe2O3NS|FePO4 | α-Fe2O3NP|FePO4 | |
| a Cathodic peak current density.b Cathodic peak potential. All parameters are determined in 0.1 M PBS solution (pH 7) containing 4.95 mM H2O2. | ||||
| Jpca (mA cm−2) | −0.996 ± 0.099 | −1.025 ± 0.035 | −1.236 ± 0.074 | −1.008 ± 0.035 |
| Epcb (V vs. Ag/AgCl) | −0.373 ± 0.026 | −0.190 ± 0.003 | −0.179 ± 0.003 | −0.345 ± 0.005 |
In a previous report,10 we proposed the EC′ mechanisms for peak r1 (eqn (4) and (5)) and peak r2 (eqn (6) and (7)):
| FePO4(s) + e− + H+ ⇄ Fe2+(ad) + HPO42− | (4) |
| Fe2+(ad) + H2O2 + HPO42− ⇄ FePO4 + H2O + OH˙ | (5) |
| OH˙ + e− → OH− | (6) |
| OH− + H+ ⇄ H2O | (7) |
The pH-insensitive reduction peak r2 was found to be related to the intrinsic catalytic properties of α-Fe2O3 itself, and an electron probably comes from the active site, that is, Fe(II) species in the electro-reduced α-Fe2O3 under cathodic conditions.15 The lack of this peak for the case of α-Fe2O3NP|FePO4 implies that this intrinsic catalytic property is structure-dependent or suppressed by other factors, such as the electrolyte or the deposited FePO4. It is worth noting that although α-Fe2O3NP|FePO4 has the highest surface area (Fig. S2†), without synergetic effects with this activity, it showed the least overall activity, in terms of Ipc and Epc, among the three α-Fe2O3NP modified electrodes. The higher overpotential required for α-Fe2O3NP|FePO4 to reduce H2O2 is in agreement with previous reports.5c,d,12a
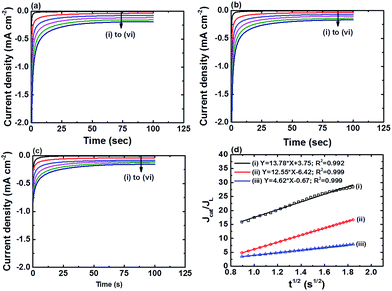
Fig. 6a–c show the chronoamperograms for all the α-Fe2O3 modified electrodes at an applied potential of −0.3 V vs. Ag/AgCl in 0.1 M PBS solution containing H2O2 of various concentration. It can be found that all the nanostructured FePO4 modified α-Fe2O3 modified electrodes exhibited catalytic current densities that are linearly proportional to the H2O2 concentration. The rate constants of the reduction of H2O2 on the nanostructured FePO4 modified α-Fe2O3 modified electrodes can be derived from Fig. 6a–c and eqn (8) and (9):16
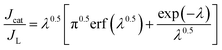 | (8) |
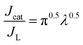 | (9) |
The values of ks for the reduction of H2O2 on α-Fe2O3NR|FePO4, α-Fe2O3NS|FePO4, and α-Fe2O3NP|FePO4, determined from the slopes of plots of Jcat/JL versus t0.5 in Fig. 6d, are found to be 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 253.9, 15
253.9, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 242.7, and 2037.9 L mol−1 s−1, respectively, which further indicates that the kinetics of the H2O2 reduction process can be further facilitated with the active Fe(II) species in α-Fe2O3. Note that although the value of ks for Fe2O3NR|FePO4 is higher than that for α-Fe2O3NS|FePO4, α-Fe2O3NS|FePO4 exhibited higher overall electrocatalytic activity over Fe2O3NR|FePO4 as α-Fe2O3NS|FePO4 has a higher surface area.
242.7, and 2037.9 L mol−1 s−1, respectively, which further indicates that the kinetics of the H2O2 reduction process can be further facilitated with the active Fe(II) species in α-Fe2O3. Note that although the value of ks for Fe2O3NR|FePO4 is higher than that for α-Fe2O3NS|FePO4, α-Fe2O3NS|FePO4 exhibited higher overall electrocatalytic activity over Fe2O3NR|FePO4 as α-Fe2O3NS|FePO4 has a higher surface area.
Fig. S5†shows CV curves of the FTO, α-Fe2O3NR, α-Fe2O3NS, and α-Fe2O3NP at a scan rate of 20 mV s−1 in 0.1 M Na2SO4 solution (pH 7) containing H2O2 of various concentration. Note that the deposition of FePO4 is impossible in this electrolyte, and the observed activity from all the α-Fe2O3 reflects their intrinsic activity. Values of Ipc and Epc of all the electrodes in the presence of 4.95 mM H2O2 are also summarized in Table S1.† As revealed, α-Fe2O3NP showed the highest apparent electrocatalytic activity, in terms of Ipc and Epc, among the four electrodes, which could be attributed to its high surface area. In addition, as shown in Fig. S6,† all the three nanostructured α-Fe2O3 modified electrodes exhibited a pH-independent current response to H2O2, which suggests the reaction catalysed by these nanostructured α-Fe2O3 should be the same, and therefore, the suppressed activity of α-Fe2O3NP|FePO4 in phosphate buffer could be attributed to the unfavourable interaction between α-Fe2O3NP and deposited FePO4 and/or phosphate ions. It has been reported that the adsorbed phosphate ions would inhibit the reduction of α-Fe2O3,17 which in turn inhibits the formation of the Fe(II) species responsible for the reduction of H2O2.
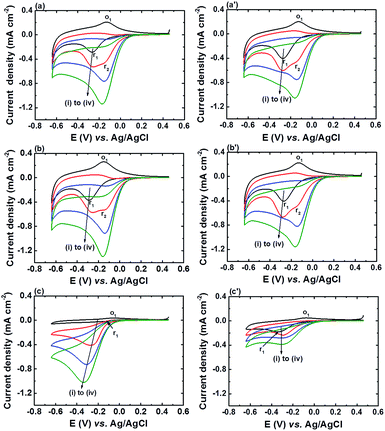
Fig. 7 shows the CV curves of α-Fe2O3NR|FePO4, α-Fe2O3NS|FePO4, and α-Fe2O3NP|FePO4 in 0.1 M PBS (pH 6) at various H2O2 concentrations under N2 and air atmospheres. The sensitivities of α-Fe2O3NR|FePO4, α-Fe2O3NS|FePO4, and α-Fe2O3NP|FePO4 obtained from the data in Fig. 7 are shown in Fig. S7.† When comparing the CV responses in the absence of H2O2 under different atmospheres, it can be found that the Ipc of peak r1 increased and the Ipa of peak o1 decreased for all the FePO4 modified α-Fe2O3 electrodes, which indicates that the deposited FePO4 is active in catalysing the electrochemical reduction of dissolved oxygen in PBS. In addition, peak r2 appeared only after the addition of H2O2 regardless of background atmosphere, which suggests that the Fe(II) sites in electro-reduced α-Fe2O3 is active for the reduction of H2O2 but not for the reduction of dissolved oxygen. As a result, the dissolved oxygen showed little effect on the sensitivity of α-Fe2O3NR|FePO4 and α-Fe2O3NS|FePO4 towards the electrochemical reduction of H2O2; the sensitivities of α-Fe2O3NR|FePO4 and α-Fe2O3NS|FePO4 towards the electrochemical reduction of H2O2 under air atmosphere remained at 96.5 ± 4.3% and 94.7 ± 4.7%, respectively, of those under N2 atmosphere. In contrast, the sensitivity of α-Fe2O3NP|FePO4 towards the electrochemical reduction of H2O2 under air atmosphere only remained at 41.4 ± 3.9% of that at N2 atmosphere. The significant influence of dissolved oxygen on α-Fe2O3NP|FePO4 can be attributed to the fact that α-Fe2O3NP|FePO4 lacks Fe(II) sites and the interaction of FePO4 with dissolved oxygen suppresses the reaction between FePO4 and H2O2. The low interference from oxygen for α-Fe2O3NR|FePO4 and α-Fe2O3NS|FePO4 makes them a potential candidate material for the detection of biomolecules involving oxidase-catalysed chemical processes.
Conclusions
α-Fe2O3 nanorods, nanosheets, and nanoparticles were successfully synthesized using chemical bath deposition and their electrocatalytic properties were thoroughly examined in phosphate buffer and non-phosphate electrolyte. In phosphate buffer, the in situ deposited FePO4 exhibited synergetic effects on the activity of α-Fe2O3 nanorods and nanosheets only, and the surface modification of FePO4 on these two nanostructured α-Fe2O3 greatly enhanced their overall electrocatalytic activity compared with FePO4 or α-Fe2O3 alone. The active sites, Fe(II), in α-Fe2O3 nanorods and nanosheets are insensitive to the dissolved oxygen, and makes them potential electrode materials for the fabrication of selective and sensitive H2O2 or related electrochemical sensors.Acknowledgements
This research was, in part, supported by the Ministry of Education, Taiwan, R.O.C. The Aim for the Top University Project to the National Cheng Kung University (NCKU). Financial support from the Ministry of Science and Technology of Taiwan (MOST 104-2221-E-006-235- and 104-ET-E-006-004-ET) are also gratefully acknowledged.Notes and references
- E. Nossol and A. J. Gorgatti Zarbin, J. Mater. Chem., 2012, 22, 1824–1833 RSC.
- J. Wang, Chem. Rev., 2008, 108, 814–825 CrossRef CAS PubMed.
- (a) J. Hrbac and R. Kohen, Drug Dev. Res., 2000, 50, 516–527 CrossRef CAS; (b) R. Kohen, E. Vellaichamy, J. Hrbac, I. Gati and O. Tirosh, Free Radical Biol. Med., 2000, 28, 871–879 CrossRef CAS.
- (a) A. A. Karyakin and E. E. Karyakina, Sens. Actuators, B, 1999, 57, 268–273 CrossRef CAS; (b) A. A. Karyakin, E. A. Puganova, I. A. Budashov, I. N. Kurochkin, E. E. Karyakina, V. A. Levchenko, V. N. Matveyenko and S. D. Varfolomeyev, Anal. Chem., 2004, 76, 474–478 CrossRef CAS PubMed.
- (a) Z. L. Liu, B. Zhao, Y. Shi, C. L. Guo, H. B. Yang and Z. A. Li, Talanta, 2010, 81, 1650–1654 CrossRef CAS PubMed; (b) L. Zhang, Y. Zhai, N. Gao, D. Wen and S. Dong, Electrochem. Commun., 2008, 10, 1524–1526 CrossRef CAS; (c) J. Hrbac, V. Halouzka, R. Zboril, K. Papadopoulos and T. Triantis, Electroanalysis, 2007, 19, 1850–1854 CrossRef CAS; (d) L. Zhang, Y. H. Ni, X. H. Wang and G. C. Zhao, Talanta, 2010, 82, 196–201 CrossRef CAS PubMed; (e) J. Gong, L. Wang, K. Zhao and D. Song, Electrochem. Commun., 2008, 10, 123–126 CrossRef CAS; (f) J. Z. Marinho, R. H. O. Montes, A. P. de Moura, E. Longo, J. A. Varela, R. A. A. Munoz and R. C. Lima, Mater. Res. Bull., 2014, 49, 572–576 CrossRef CAS.
- (a) C.-Y. Lin, Y.-H. Lai, A. Balamurugan, R. Vittal, C.-W. Lin and K.-C. Ho, Talanta, 2010, 82, 340–347 CrossRef CAS PubMed; (b) S. Wu, H. Zhao, H. Ju, C. Shi and J. Zhao, Electrochem. Commun., 2006, 8, 1197–1203 CrossRef CAS.
- (a) K. Schachl, H. Alemu, K. Kalcher, J. Jezkova, I. Svancara and K. Vytras, Anal. Lett., 1997, 30, 2655–2673 CrossRef CAS; (b) J.-H. Lee and H.-G. Hong, J. Appl. Electrochem., 2015, 45, 1153–1162 CrossRef CAS.
- (a) X.-M. Miao, R. Yuan, Y.-Q. Chai, Y.-T. Shi and Y.-Y. Yuan, J. Electroanal. Chem., 2008, 612, 157–163 CrossRef CAS; (b) J. M. Zen, H. H. Chung and A. S. Kumar, Analyst, 2000, 125, 1633–1637 RSC.
- L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang, S. Perrett and X. Yan, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef CAS PubMed.
- C.-Y. Lin and C.-T. Chang, Sens. Actuators, B, 2015, 220, 695–704 CrossRef CAS.
- (a) M. Magro, D. Baratella, G. Salviulo, K. Polakova, G. Zoppellaro, J. Tucek, J. Kaslik, R. Zboril and F. Vianello, Biosens. Bioelectron., 2014, 52, 159–165 CrossRef CAS PubMed; (b) M. Magro, D. Baratella, N. Pianca, A. Toninello, S. Grancara, R. Zboril and F. Vianello, Sens. Actuators, B, 2013, 176, 315–322 CrossRef CAS; (c) D. Baratella, M. Magro, G. Sinigaglia, R. Zboril, G. Salviulo and F. Vianello, Biosens. Bioelectron., 2013, 45, 13–18 CrossRef CAS PubMed; (d) A. K. Dutta, S. K. Maji, D. N. Srivastava, A. Mondal, P. Biswas, P. Paul and B. Adhikary, J. Mol. Catal. A: Chem., 2012, 360, 71–77 CrossRef CAS.
- (a) J. M. Gong, L. Y. Wang, K. Zhao and D. D. Song, Electrochem. Commun., 2008, 10, 123–126 CrossRef CAS; (b) S. Nath, C. Kaittanis, V. Ramachandran, N. S. Dalal and J. M. Perez, Chem. Mater., 2009, 21, 1761–1767 CrossRef CAS PubMed; (c) S. H. Liu, F. Lu, R. M. Xing and J. J. Zhu, Chem.–Eur. J., 2011, 17, 620–625 CrossRef CAS PubMed; (d) N. Puvvada, P. K. Panigrahi, D. Mandal and A. Pathak, RSC Adv., 2012, 2, 3270–3273 RSC.
- S. Trasatti and O. A. Petrii, Pure Appl. Chem., 1991, 63, 711–734 CrossRef CAS.
- (a) F. Marken, D. Patel, C. E. Madden, R. C. Millward and S. Fletcher, New J. Chem., 2002, 26, 259–263 RSC; (b) K. J. McKenzie and F. Marken, Pure Appl. Chem., 2001, 73, 1885–1894 CrossRef CAS.
- M. S. Lin and H. J. Len, Electroanalysis, 2005, 17, 2068–2073 CrossRef CAS.
- F. Pariente, E. Lorenzo, F. Tobalina and H. D. Abruna, Anal. Chem., 1995, 67, 3936–3944 CrossRef CAS.
- C. Liu, J. M. Zachara, N. S. Foster and J. Strickland, Environ. Sci. Technol., 2007, 41, 7730–7735 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/c6ra07267d |
| This journal is © The Royal Society of Chemistry 2016 |