Magnetically separable Fe3O4@chitin as an eco-friendly nanocatalyst with high efficiency for green synthesis of 5-substituted-1H-tetrazoles under solvent-free conditions†
Monireh
Zarghani
and
Batool
Akhlaghinia
*
Department of Chemistry, Faculty of Sciences, Ferdowsi University of Mashhad, Mashhad 9177948974, Iran. E-mail: akhlaghinia@um.ac.ir; Tel: +98-51-3880-5527
First published on 24th March 2016
Abstract
The present study describes an efficient, eco-friendly and simple method for the synthesis of 5-substituted-1H-tetrazoles catalyzed by magnetite–chitin (Fe3O4@chitin) as a green and recyclable catalyst. Fe3O4@chitin was initially prepared using hydrothermal synthesis. Subsequently, the structure, morphology, and magnetic properties of the prepared nanocatalyst were studied with some different spectroscopic, microscopic and thermogravimetric techniques such as FT-IR, XRD, SEM, TEM, VSM and TGA. Obtained results showed that Fe3O4@chitin nanoparticles exhibited uniform cubic shape and were well monodispersed. Also, magnetic measurement revealed that the synthesized nanocatalyst had superparamagnetic features. The application of this new nanocatalyst allows the synthesis of a variety of tetrazoles through the reaction of nitriles with 1-butyl-3-methylimidazolium azide ([bmim][N3]) under solvent-free conditions. This synthetic pathway is a green protocol offering significant advantages, such as excellent yield of products in short reaction times, mild reaction conditions, minimization of chemical waste, easy preparation of the catalyst and its recyclability up to six cycles without any considerable loss of efficiency.
1. Introduction
During recent decades, there has been considerable research interest in the area of green chemistry using environmentally benign reagents and clean synthetic procedures. In this context, nanotechnology, as one of the most fascinating developments, can be applied to produce safer and more sustainable nanostructured materials and more efficient chemical processes.1–5 Among the various nanomaterial, magnetic nanoparticles as an eco-friendly metal oxide, have received considerable attention because of their potential applications, including magnetic fluids,6 magnetic resonance imaging,7 biotechnology/biomedicine8 and data storage.9 Moreover, recent search has been shown that magnetic nanoparticles could be efficient and promising supports for catalysts.10–15 From the viewpoint of green chemistry, it is useful to develop new catalyst recycling methods to replace conventional procedures such as centrifugation and filtration.16 Thus, magnetite nanoparticles became the strong candidate due to unique paramagnetic properties which allow them to be efficiently separated from the reaction mixtures by an external magnet.17–20 Additionally, other important features of these nanoparticles are high degree of chemical stability in various solvents, easy synthesis and modification, high surface area, low toxicity, cost-effective and benign character.21On the other hand, the recent scientific reports indicate that natural polymers with many significant biological (biodegradable, biocompatible and bioactive) and chemical properties (polycationic, hydrogel, reactive groups such as OH and NH2) can be used as heterogeneous catalytic systems.22–25 Chitin as a linear aminopolysaccharide to be found as skeletal scaffold in broad variety of fungi, protists, diatoms and invertebrates species,26 is one of the most abundant biopolymer on earth after cellulose. This biopolymer has been proven to be an extremely promising material because of its many advantageous characteristics such as biodegradability, biocompatibility, excellent chelation behavior, and the least toxicity.27 Chitin composed of 2-acetamide-2-deoxy-D-glucopyranose, has highly crystalline structure due to strong hydrogen bonding of two hydroxyl and an acetamide groups in its linear structure. In addition, it has been found that hydroxyl groups and N-acetyl amide group in the glucose ring of chitin have the excellent chelation capacity with metal ions.28
Other important feature of this biopolymer is high thermal stability up to 360 °C. Literature survey shows that chitin is a stable biopolymer under common hydrothermal conditions and could be effectively used as a scaffold for hydrothermal mineral deposition. In this regard, there have been a variety of reports describing the use of chitin as a template for the hydrothermal synthesis of nanocrystals such as ZnO, ZrO2, SiO2, and Fe2O3 (hematite), GeO2.29–33
Recently, the chemistry of heterocycles has acquired immense importance. Among this, 5-substituted-1H-tetrazoles which represent an important class of heterocyclic compounds have attracted great attention in recent years due to their wide range of applications. Most important applications of these compounds include their use in pharmaceuticals, material sciences, coordination chemistry and recognized as a useful intermediate in synthetic organic chemistry.34–38 Up to now, numerous methods have been reported for the preparation of 5-substituted-1H-tetrazoles in the presence of various reactants and catalysts,39–43 but each has its own disadvantage, such as the use of strong Lewis acids, expensive reagents, longer reaction times, tedious separation procedures and use of large amount of toxic solvents. Therefore, it is of great practical importance to develop a more efficient and environmentally benign method that avoids these drawbacks.
Consequently, due to our interest towards the extension of environmentally friendly methods for the synthesis of tetrazoles44–47 and based on our previous experience in this respect (that hydrogen bonding of chitin could be catalyzed this cycloaddition reaction),46 we decided to prepare and characterize magnetite–chitin nanocatalyst as a novel, green and recyclable heterogeneous catalyst (Scheme 1). Then synthesis of tetrazoles via reaction between nitriles and azide ion was examined in solvent free condition. 1-Butyl-3-methylimidazolium azide [bmim][N3] was used as the azide ion source in the present study. The results showed that magnetite–chitin nanocatalyst is highly efficient in this cycloaddition reaction, and it can be easily separated from the reaction mixture.
2. Experimental
2.1. Chemicals and instruments
All chemical reagents and solvents were purchased from Merck and Sigma-Aldrich chemical companies and were used as received without further purification. Chitin was extracted from cuttlebone which was taken out from cuttlefish (Sepia esculenta).46 It is commonly found in saltwater beaches like Persian Gulf in Iran.The purity determinations of the products were accomplished by TLC on silica gel polygram STL G/UV 254 plates. The melting points of products were determined with an Electrothermal Type 9100 melting point apparatus. The FTIR spectra were provided on pressed KBr pellets using an AVATAR 370 FT-IR spectrometer (Therma Nicolet spectrometer, USA) at room temperature in the range between 4000 and 400 cm−1. The NMR spectra were obtained in Brucker Avance 100, 300 and 400 MHz instruments in CDCl3, DMSO-d6 and CD3CN. Elemental analysis was performed using a Thermo Finnigan Flash EA 1112 Series instrument. Mass spectra were recorded with a CH7A Varianmat Bremem instrument at 70 eV electron impact ionization, in m/z (rel%). TGA analysis was carried out on a Shimadzu Thermogravimetric Analyzer (TG-50) in the temperature range of 25–900 °C at a heating rate of 10 °C min−1 under air atmosphere. Transmission electron microscopy (TEM) was performed with a Leo 912 AB microscope (Zeiss, Germany) with an accelerating voltage of 120 kV. SEM images were also recorded using Leo 1450 VP scanning electron microscope operating at an acceleration voltage 20 kV. The crystal structure of catalyst was analyzed by XRD using a D8 ADVANCE-Bruker diffractometer operated at 40 kV and 30 mA utilizing CuKα radiation (λ = 0.154 Å). The magnetic property of catalyst was measured using a vibrating sample magnetometer (VSM, 7400 Lake Shore). Inductively-coupled plasma mass spectrometry (ICP-MS) was carried out on a Varian Australia. All of the products were known compounds and they were characterized by the FT-IR spectroscopy, 1H NMR, 13C NMR spectroscopy, and mass spectrometry and comparison of their melting points with known compounds.
2.2. Extraction of β-chitin from cuttlebone
A cuttlebone (≈15 g) was cut into 1 × 1 cm pieces and soaked for 12 h in 500 mL of 2 M HCl under low vacuum. A low vacuum was used to exclude CO2 bubbles, which formed during dissolution of the aragonite component, from the internal chambers of the organic matrix. The procedure was repeated overnight in fresh aqueous 2 M HCl. The pieces were then washed with distilled water until neutral pH and added to 500 mL of 1 M NaOH at a boiling temperature for 4 h to remove associated proteins. The samples were filtered, washed with copious amounts of water, and added to 95% ethanol for 3 h, followed by further washing with distilled water. The organic matrix was stored in water at 4 °C.27a2.3. Hydrothermal synthesis of the Fe3O4@chitin
Into a three-necked 500 mL round-bottom flask equipped with argon gas inlet tube and dropping funnel, 2 g of chitin was dispersed in 150 mL of deionized water. Then, FeCl3·6H2O (46 mmol, 12.4 g) and FeSO4·7H2O (23 mmol, 6.3 g) were added to the white suspension. The resulting mixture was mechanically stirred for 3 min at room temperature under Ar atmosphere. Consequently, 20 mL ammonium hydroxide (25%) was dropped very slowly into the mixture under vigorous stirring. The resulting black mixture was continuously stirred for 5 h at 75 °C under Ar atmosphere. Finally, the mixture was permitted to cool at room temperature and Fe3O4@chitin nanoparticles were separated by an external magnet and washed with deionized water several times before being dried under vacuo at 50 °C overnight.2.4. Synthesis of 1-butyl-3-methylimidazolium chloride
1-Chlorobutane (10.8 g, 0.8 mol) was added to 1-methyimidizole (8.2 g, 0.1 mol), in a round bottomed flask equipped with a reflux condenser. The mixture was stirred at 70 °C for 48 h. After formation of two phases, the top layer which contains unreacted starting material was decanted. Then ethyl acetate (30 mL) was added with entire mixing followed by its decantation and this step was repeated three times. After the third decanting of ethyl acetate, remaining solvent was removed by heating at 70 °C. The obtained pale yellow liquid was vacuum distilled and the resulting 1-butyl-3-methylimidazolium chloride was placed for vacuum drying at 80 °C in a vacuum drying oven.482.5. Synthesis of 1-butyl-3-methylimidazolium azide
Freshly prepared 1-butyl-3-methylimidazolium chloride (3.49 g, 20 mmol) and NaN3 (1.30 g, 20 mmol) were added into 25 mL deionized water, and the mixture was stirred for 24 h at room temperature. The solvent was removed under reduced pressure at 50 °C. To separate 1-butyl-3 methylimidazolium azide from the crude product (which contains azide ionic liquid and NaCl), the resulting mixture was washed with acetonitrile (3 × 10 mL).The remaining acetonitrile was removed under high vacuum to yield yellow transparent liquid that became more viscous upon extensive drying. Isolated yield was 92% (3.33 g).49
2.6. Typical procedure for synthesis of 5-phenyl-1H-tetrazole in the presence of the Fe3O4@chitin
A mixture of benzonitrile (0.103 g, 1 mmol) and [bmim][N3] (0.216 g, 1.2 mmol) in the presence of 0.03 g of Fe3O4@chitin was stirred at 110 °C. The progress of the reaction was monitored by TLC. When the reaction was completed, the catalyst was removed magnetically and then 10 mL HCl (4 N) was added to the resulting solution. Finally, the obtained aqueous solution was extracted with ethyl acetate (2 × 10 mL) and the combined organic phase was washed with distilled water (2 × 20 mL), dried over anhydrous sodium sulfate and concentrated to obtain the white precipitate. The crude 5-phenyl-1H-tetrazole was recrystallized from n-hexane/ethylacetate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) obtaining 0.1386 g of colourless crystals (95% yield).
1) obtaining 0.1386 g of colourless crystals (95% yield).
3. Results and discussion
3.1. Characterization of catalyst
The Fe3O4@chitin as a magnetically heterogeneous nanocatalyst was prepared by hydrothermal method which consists of the reaction between Fe2+ and Fe3+ ions and NH4OH in the presence of chitin (see Scheme 1). The composition of the synthesized nanocatalyst was analyzed by some technical methods such as Fourier transform infrared (FT-IR) spectroscopy, X-ray diffraction (XRD), transmission electron microscopy (TEM), scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDX), thermogravimetric analysis (TGA) and vibrating sample magnetometry (VSM).FT-IR spectroscopy is an appropriate technique to identify surface chemical structure of nanocatalyst. The FT-IR spectra of chitin as well as the magnetite–chitin nanocatalyst in the range of 4000–400 cm−1 have been depicted in Fig. 1a and b respectively. The FT-IR spectrum of chitin was established by the following absorption bands: O–H and N–H stretching vibrations around 3460–3250 cm−1, C–H stretching vibrations at 2977 and 2870 cm−1, N–H bending vibrations at 1680 cm−1. Furthermore, the specific amide bonds were detected at 1662 cm−1 (amide I), 1550 cm−1 (amide II) and 1315 cm−1 (amide III). The intense band located at 1475 cm−1 (C–N stretching vibrations) and the broad bands ranging from 1160 to 1010 cm−1 (C–O–C and C–O stretching vibrations) are indicative of chitin. In addition, the other strong chitin band at 860 cm−1 (related to –CH3 in acetylamide groups) can be clearly observed in Fig. 1a.50
The FT-IR spectrum of Fe3O4@chitin was shown as Fig. 1b. As can be seen, the presence of Fe3O4 nanoparticles was confirmed by detecting absorption band at around 603 cm−1 which corresponding to the Fe–O stretching bond of magnetite nanoparticles.51 On the other hand, although the all characteristic bands of chitin were present in the FT-IR spectrum of nanocatalyst but some of them exhibited noticeable changes.52 For instance, after incorporation of magnetite in the chitin matrix, hydroxyl and acetyl groups frequencies (which related to absorption bands appearing around 3460 and 3250 cm−1 in chitin) shifted to 3420 and 3260 cm−1 in Fe3O4@chitin, respectively. Its occurrence may indicate an interaction between the Fe3O4 nanoparticles and the –OH and –NH stretching vibration of chitin. Splitting of the absorption bands at 1620 (amide I) and 1550 cm−1 (amide II) (due to –NH bending vibrations) and 857–876 cm−1 (–CH3 in acetylamide groups) also confirmed these interactions. Thus, overall the observation revealed that Fe3O4@chitin as a green nanocatalyst was successfully synthesized via an environmentally friendly process.
Also, XRD analysis was applied to detect the crystallinity of the synthesized nanocatalyst (Fig. 2). As shown in Fig. 2a, the characteristic peaks with strong intensities appeared at angles corresponding to (2, 2, 0), (3, 1, 1), (4, 0, 0), (4, 2, 2), (5, 1, 1) and (4, 4, 0) reflections were in good agreement with the standard pattern for crystalline magnetite with cubic structure (JCPDS 19-0629).53 Moreover, in comparison with the standard pattern of pure Fe3O4 MNPs, two additional small and weak peaks (at 9.6 and 19.6 angles) were found in the XRD pattern of Fe3O4@chitin which could be attributed to the characteristic peaks of chitin (Fig. 2b).54
This fact suggested that the coating process did not result in the phase change of Fe3O4 nanoparticles.
To elucidate the morphology and size of the synthesized nanocatalyst, Fe3O4@chitin was analyzed by SEM and TEM (Fig. 3). It is evident from SEM image (Fig. 3a) that the shape of Fe3O4@chitin nanocatalyst is not clearly defined, however, synthesized nanocatalyst is well dispersed. The TEM micrograph of the Fe3O4@chitin shown in Fig. 3b revealed that the synthesized nanoparticles existed as cubicles with a mean edge length of 25 nm and were nearly monodisperse.55 Obtained results indicated that the presence of chitin during the synthesis of Fe3O4 nanoparticles, leads to improvement of the nanoparticles size and distribution. Energy-dispersive X-ray analysis (EDX), indicated the presence of C, O and Fe elements (Fig. 4), which further supported the conclusion that Fe3O4 nanoparticles have been synthesized successfully in the presence of chitin.
Thermogravimetric analysis (TGA) of the Fe3O4@chitin was also used to determine the content of organic materials of the nanocatalyst, as shown in Fig. 5. Upon heating, Fe3O4@chitin showed a weight loss of about 6.24% at temperatures ranging from 50 to 140 °C, mainly due to the loss of water, because polysaccharides usually have a strong affinity for water and therefore may be easily hydrated. The second weight loss (3.02% in the range of 160–340 °C) was attributed to the partial decomposition of chitin chain. After that, there was a significant weight loss (about 9.01%) from 350 °C to 650 °C corresponding to the complete decomposition of biopolymer backbone. In comparison with literature,27b,29–33 it can be concluded that chitin has somehow greater stability due to interaction with Fe3O4 nanoparticles.
The magnetic property of Fe3O4@chitin, which was critical to ensure its application, was measured using VSM. The hysteresis loop of synthesized nanocatalyst is shown in Fig. 6. The results of VSM showed that the magnetic saturation value of Fe3O4@chitin was 32.5 emu g−1, which was lower than the reference value for pure Fe3O4 MNPs.53 It may be ascribed to the cover of chitin polymeric matrix on the surface of magnetic Fe3O4 MNPs. Moreover, it is clearly seen from this figure that no remanence and coercivity was observed, which indicated that the nanoparticles obtained in this study are superparamagnetic. These magnetic properties allow easily and quickly separation of the nanocatalyst from the reaction mixture with the help of an external magnet.
 | ||
| Fig. 6 (a) Magnetization curve of Fe3O4@chitin (b) separation of nanocatalyst using an external magnet. | ||
3.2. Catalytic synthesis of 5-substituted-1H-tetrazoles
Recently, we reported an efficient and green synthesis of 5-substituted-1H-tetrazoles from organic nitriles and azide ion using highly porous expanded perlite as a natural heterogeneous catalyst in high yields.47 Accordingly, 1-butyl-3-methylimidazolium azide ([bmim][N3]) ionic liquid was used as an azide ion source instead of the highly toxic reagents such as NaN3 or TMSN3. Also, the cycloaddition reaction was performed in solvent free condition that in comparison with the most reported literature, it was very eco-friendly procedure. These advantages become even more attractive if such reactions can be catalyzed by using easily recyclable catalyst such as magnetic nanoparticles. Hence, according to our previous experience and in order to extend the range of high efficient heterogeneous catalyst, we now report a new method for the preparation of 5-substituted-1H-tetrazoles via reaction between a wide variety of nitriles and [bmim][N3] as an azide source in solvent free condition by using Fe3O4@chitin as a simple, green, and recyclable catalyst (Scheme 2).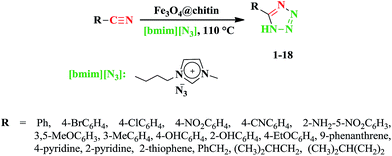 | ||
| Scheme 2 Synthesis of 5-substituted-1H-tetrazoles in the presence of Fe3O4@chitin under solvent-free conditions. | ||
To obtain the best conditions for the synthesis of 5-substituted-1H-tetrazoles, initial studies were performed upon the reaction of benzonitrile with 1-butyl-3-methylimidazolium azide as a model reaction and the effects of different conditions were investigated for this reaction (Table 1). According to Table 1, when the model reaction was performed under catalyst-free conditions at 100 °C, the desired product was obtained in low yield (Table 1, entry 1), whereas, using 0.03 g of Fe3O4@chitin in [3 + 2] cycloaddition reaction as a nanocatalyst afforded high yield of the product (90%) in short reaction time (20 min) (Table 1, entry 2). To study the effect of different molar ratios of reactants, [3 + 2] cycloaddition reaction was performed with different molar ratios of benzonitrile/[bmim][N3] (Table 1, entries 2, 3 and 4). As shown, increasing the amount of [bmim][N3] was found to have no effect on the yield (90%) of [3 + 2] cycloaddition reaction. In contrast, decreasing the amount of [bmim][N3] dropped down the yield reaction a lot. The effect of temperature on the reaction was also evaluated (Table 1, entries 5, 6). The best result was achieved by carrying out the reaction at 110 °C, affording 95% of 5-phenyl-1H-tetrazole in 20 min. Different amount of the catalyst (0.035, 0.03 and 0.02 g) was also optimized and it was found that 0.03 g of catalyst gave the maximum yield of the product (Table 1, entries 7, 8). An increase in the amount of catalyst did not improve the results to any great extent. However using 0.02 g of catalyst could not provide satisfactory yield. Comprehensively, the optimum reaction conditions for synthesis of 5-phenyl-H-tetrazole were as follows: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 molar ratio of reactants at 110 °C in the presence of 0.03 g of Fe3O4@chitin as catalyst, under solvent free conditions. Consequently, we checked the effect of Fe3O4 nanoparticles and chitin on the [3 + 2] cycloaddition reaction, by carrying out the reaction under the optimized reaction conditions, separately (Table 1, entries 9 and 10). It was observed that, by using 0.03 g of Fe3O4 nanoparticles as catalyst, the desired product was obtained in low yield and in long reaction time (50%, 1 h), while, the same reaction in the presence of 0.03 g chitin gave admirable yield (70%) in short reaction time (30 min) (Table 1, entry 10). In comparison with catalytic performance of Fe3O4@chitin (95% yield in 20 min vs. 70% in 30 min), it could be concluded that Lewis acidity of Fe3O4 nanoparticles may promote the [3 + 2] cycloaddition reaction.56 However, it should be mentioned that the aim of supporting chitin on the surface of magnetic nanoparticles was its easy magnetically separation, its recovery and its reusability.
1.2 molar ratio of reactants at 110 °C in the presence of 0.03 g of Fe3O4@chitin as catalyst, under solvent free conditions. Consequently, we checked the effect of Fe3O4 nanoparticles and chitin on the [3 + 2] cycloaddition reaction, by carrying out the reaction under the optimized reaction conditions, separately (Table 1, entries 9 and 10). It was observed that, by using 0.03 g of Fe3O4 nanoparticles as catalyst, the desired product was obtained in low yield and in long reaction time (50%, 1 h), while, the same reaction in the presence of 0.03 g chitin gave admirable yield (70%) in short reaction time (30 min) (Table 1, entry 10). In comparison with catalytic performance of Fe3O4@chitin (95% yield in 20 min vs. 70% in 30 min), it could be concluded that Lewis acidity of Fe3O4 nanoparticles may promote the [3 + 2] cycloaddition reaction.56 However, it should be mentioned that the aim of supporting chitin on the surface of magnetic nanoparticles was its easy magnetically separation, its recovery and its reusability.
| Entry | Molar ratio (benzonitrile/[bmim][N3]) | Catalyst (g) | Temperature (°C) | Time (min) | Isolated yield (%) |
|---|---|---|---|---|---|
| a The reaction was performed in the presence of Fe3O4 NPs. b The reaction was performed in the presence of chitin. | |||||
| 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
— | 100 | 1 (h) | 10 |
| 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
0.03 | 100 | 20 | 90 |
| 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
0.03 | 100 | 20 | 90 |
| 4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.03 | 100 | 20 | 70 |
| 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
0.03 | 120 | 20 | 95 |
| 6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
0.03 | 110 | 20 | 95 |
| 7 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
0.02 | 110 | 20 | 85 |
| 8 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
0.035 | 110 | 20 | 95 |
| 9a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
0.03 | 110 | 1 (h) | 50 |
| 10b | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
0.03 | 110 | 30 | 70 |
To assess the efficiency and scope of the present methodology, various 5-substituted-1-H-tetrazoles were synthesized by the [3 + 2] cycloaddition reaction between a wide range of structurally divergent nitriles and [bmim][N3] in presence of Fe3O4@chitin under the optimized reaction conditions. The representative results are summarized in Table 2. Generally, the aromatic nitriles containing electron withdrawing and electron-donating substituent were well tolerated under the reaction conditions and gave the corresponding tetrazoles in good to excellent yields. However, as it is shown in Table 2, reactions of electron poor nitrile compounds were completed within a short reaction time, whereas electron rich nitriles required more reaction time to proceed properly (compare entries 1–5 with entries 6–11). So, it can be concluded that the electronic character of substituents on the aromatic rings of the nitriles had a noteworthy effect on the efficiency of the reaction which demonstrated that electron donating substituents on aromatic rings decrease the electrophilic character of nitrile. In the same way, the reaction was examined using phenanthrene-9-carbonitrile in which the desired product was obtained in good yield, although longer reaction time was required which may be due to the steric hindrance (Table 2 entry 12).
Furthermore, when heteroaromatic nitriles such as thiophene-2-carbonitrile, 4-pyridinecarbonitrile and 2-pyridinecarbonitrile were used as substrate, due to the strong electron withdrawing effect of the heteroatoms, excellent yields of the corresponding 5-substituted-1-H-tetrazoles were obtained in a short reaction time (Table 2 entries 13, 14 and 15). It is noteworthy that not only aromatic nitriles but also aliphatic ones could also be employed to afford the desired products in admirable yields (Table 2 entries 16, 17 and 18). These results demonstrated the superior properties of Fe3O4@chitin as an eco-friendly and effective heterogeneous catalysts for the synthesis of 5-substituted-1-H-tetrazoles by [3 + 2] cycloaddition from nitriles and [bmim][N3].
In order to establish the structure of synthesized tetrazoles, their melting points were initially compared with the known compounds. The data were found to be in good agreement with expected values. Then to further elucidate the structure of these compounds, spectroscopic methods such as FT-IR spectroscopy, mass spectrometry, 1H NMR and 13C NMR spectroscopy were recorded. Key features in the FT-IR spectra of the products include the absorption bands at: 3485–3329 cm−1 (related to the N–H stretching vibration), 1469–1430 cm−1 (due to scissoring bending C–H), 1293–1233 cm−1 (due to N–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 1189–1110 and 1106–1041 cm−1 (due to tetrazole ring). Also, in 1HNMR and 13C NMR spectra, a signal at 10.98–7.80 and 164–151 ppm which correspond to the NH and quaternary carbon NH–C
N–), 1189–1110 and 1106–1041 cm−1 (due to tetrazole ring). Also, in 1HNMR and 13C NMR spectra, a signal at 10.98–7.80 and 164–151 ppm which correspond to the NH and quaternary carbon NH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, respectively, can be beneficial for further confirming the structure of desired products.
N, respectively, can be beneficial for further confirming the structure of desired products.
The catalytic activity of Fe3O4@chitin in [3 + 2] cycloaddition reaction was proved by the fact that a long reaction time and comparatively lower yield of product was observed when the model reaction was carried out in absence of Fe3O4@chitin which do not afford formation of desired product (Table 2, entry 1). Based on the literature, as well as our findings, plausible reaction pathway is shown in Scheme 3.46,57 Initially, we assume that nitrile functionality is activated through hydrogen bond formation between nanocatalyst hydroxyls and nitrogen atom of nitrile group, so facilitate the attack of azide ion which leads to the formation of intermediate I. In the next step, the intermediate II is afforded from the [3 + 2] cycloaddition reaction between activated nitrile and azide ion. At last, protonolysis of the intermediate II by HCl (4 N) causes to produce III and IV. However, the more stable tautomer IV is achieved due to the equilibrium (5-substituted 1H-tetrazole). The nanocatalyst re-enters to the catalytic cycle by making the active sites for further turnovers.
 | ||
| Scheme 3 Plausible mechanism for the formation of 5-substituted-1H-tetrazoles in presence of Fe3O4@chitin. | ||
One of the main advantages associated with supporting a catalyst onto Fe3O4 MNPs is the possibility of recovery by simple magnetic decantation and reuse without loss of activity. So, in relevance with this, the activity of the recycled catalyst was examined using the reaction of benzonitrile and [bmim][N3] under the optimized conditions. In order to regenerate the nanocatalyst, upon completion of reaction, it was separated magnetically and washed several times with deionized water and ethyl acetate to remove residual compounds. The resultant nanocatalyst was dried at 50 °C under vacuum, and reused for subsequent runs. As shown in Fig. 7, the recycled catalyst could be reused up to six runs without any noteworthy loss of activity, which demonstrates the practical recyclability of the synthesized nanocatalyst. Moreover, in order to investigate the amount of Fe leaching in these reactions, after magnetically separation of the nanocatalyst, the resulting mixture was analyzed by ICP-MS before and after the six cycles. The results showed the absence of Fe in the first run, while a very small amount of Fe (0.0021 mmol) was detected after six runs. It indicates that 95% of the nanocatalyst could be recovered in this method.
In addition, a comparative study of the reported methods in the literature on [3 + 2] cycloaddition reaction was compiled in Table 3 to highlight the advantages of this nanocatalyst. Table 3 clearly demonstrates that lower temperature and shorter reaction time were required for this cycloaddition reaction, while, several of the previously reported procedures did not provide these conditions.58–62 However, recently, we have been succeeded to perform this [3 + 2] cycloaddition reaction in a very short time by applying new natural heterogeneous catalyst.46,47 Similarly, the catalytic system presented in the present study offers excellent yields in short reaction times with the difference that the Fe3O4@chitin can be easily separated from the reaction mixture by using an external magnet.
| Entry | Catalyst | Solvent | Temperature (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|---|
| 1 | Fe3O4@SiO2/salen Cu(II)58 | DMF | 120 | 7 | 90 |
| 2 | CuFe2O4 (ref. 59) | DMF | 120 | 12 | 82 |
| 3 | Ag NPs60 | DMF | 120 | 8 | 92 |
| 4 | Cyanuric chloride61 | DMF | 120 | 2 | 90 |
| 5 | CAN/HY-zeolite62 | DMF | 110 | 4 | 93 |
| 6 | CuSO4·5H2O44 | DMSO | 140 | 1 | 98 |
| 7 | CAES45 | DMSO | 130 | 1 | 95 |
| 8 | Cuttlebone46 | DMSO | 120 | 20 (min) | 98 |
| 9 | Expanded perlite47 | Solvent-free | 110 | 10 (min) | 95 |
| 10 | Fe3O4@chitin | Solvent-free | 110 | 20 (min) | 95 |
Additionally, this nanocatalyst promoted the synthesis of 5-substituted-1H-tetrazole in solvent-free conditions instead of toxic solvents, which follows along the line of green chemistry.
4. Conclusions
In summary, with the aim of have a good contribution in innovation of green chemistry, we have reported an operationally simple, clean and highly efficient procedure for the synthesis of 5-substituted-1H-tetrazoles using Fe3O4@chitin nanocatalyst in a green media. As a novel and eco-friendly heterogeneous catalyst, Fe3O4@chitin was easily prepared and characterized by spectroscopic, magnetic and thermal techniques (TEM, SEM, FT-IR, XRD, VSM and TGA). The ultimate material was found to be Fe3O4@chitin nanocatalyst with a mean edge length of 25 nm, cubic shape, and strong magnetic property. Then synthesized nanocatalyst has been used as an efficient catalyst for the preparation of various 5-substituted-1-H-tetrazoles via reaction between nitriles and [bmim][N3] as the azide ion source under solvent-free condition. Excellent results were obtained in each case affording the corresponding tetrazoles in short reaction times and high yields. Furthermore, this process avoids problems associated with organic solvent and toxic reagent use, which makes it a useful and attractive protocol in view of the economic and environmental advantages. Apart from this, simplicity of product separation and effortless reusability of nanocatalyst are the important features of this method.Acknowledgements
The authors gratefully acknowledge the partial support of this study by Ferdowsi University of Mashhad Research Council (Grant no. p/3/29760).Notes and references
- A. Benhammou, A. Yaacoubi, L. Nibou and B. Tanouti, J. Colloid Interface Sci., 2005, 282, 320 CrossRef CAS PubMed.
- M. Prasad and S. Saxena, Ind. Eng. Chem. Res., 2004, 43, 1512 CrossRef CAS.
- C. C. Liu, M. K. Wang and Y. S. Li, Ind. Eng. Chem. Res., 2005, 44, 1438 CrossRef CAS.
- A. Özcan, A. S. Özcan, S. Tunali, T. Akar and I. Kiran, J. Hazard. Mater., 2005, 124, 200 CrossRef PubMed.
- G. F. Goya, T. S. Berquo and F. C. Fonseca, J. Appl. Phys., 2003, 94, 3520 CrossRef CAS.
- S. Chikazumi, S. Taketomi, M. Ukita, M. Mizukami, H. Miyajima, M. Setogawa and Y. Kurihara, J. Magn. Magn. Mater., 1987, 65, 245 CrossRef CAS.
- Y. M. Huh, Y. W. Jun, H. T. Song, S. Kim, J. S. Choi, J. H. Lee, S. Yoon, K. S. Kim, J. S Shin, J. S. Suh and J. Cheon, J. Am. Chem. Soc., 2005, 127, 12387 CrossRef CAS PubMed.
- (a) Q. A. Pankhurst, BT Technology Journal, 2006, 24, 33 CrossRef; (b) Z. P. Xu, Q. H. Zeng, G. Q. Lu and A. B. Yu, Chem. Eng. Sci., 2006, 61, 1027 CrossRef CAS.
- D. L. Huber, Small, 2005, 1, 482 CrossRef CAS PubMed.
- M. Ma, Q. Zhang, D. Yin, J. Dou, H. Zhang and H. Xu, Catal. Commun., 2012, 17, 168 CrossRef CAS.
- F. Zamani and S. M. Hosseini, Catal. Commun., 2014, 43, 164 CrossRef CAS.
- Y. Q. Zhang, X. W. Wei and R. Yu, Catal. Lett., 2010, 135, 256 CrossRef CAS.
- M. B. Gawande, P. S. Brancoa and R. S. Varma, Chem. Soc. Rev., 2013, 42, 3371 RSC.
- Z. Wang, P. Xiao, B. Shen and N. He, Colloids Surf., A, 2006, 276, 116 CrossRef CAS.
- V. Polshettiwar, B. Baruwati and R. S. Varma, Green Chem., 2009, 11, 127 RSC.
- W. Zhang, X. Chen, T. Tang and E. Mijowska, Nanoscale, 2014, 6, 12884 RSC.
- Y. Liu, M. Chen and Y. Hao, Chem. Eng. J., 2013, 218, 46 CrossRef CAS.
- R. Hudson, Y. Feng, R. S. Varmab and A. Moores, Green Chem., 2014, 16, 4493 RSC.
- C. Amatore, S. Bensalen, S. Ghalem and A. Jutand, J. Organomet. Chem., 2004, 689, 4642 CrossRef CAS.
- C. Amatore and A. Jutand, J. Organomet. Chem., 1999, 576, 254 CrossRef CAS.
- A. Schatz, M. Hager and O. Reiser, Adv. Funct. Mater., 2009, 19, 2109 CrossRef.
- Z. Zarnegar and J. Safari, RSC Adv., 2014, 4, 20932 RSC.
- G. Li, Y. Jiang, K. Huang, P. Ding and J. Chen, J. Alloys Compd., 2008, 466, 451 CrossRef CAS.
- M. R. Shushizadeh, E. Moghimi Pour, A. Zare and Z. Lashkari, Bioact. Carbohydr. Diet. Fibre, 2015, 6, 133 CrossRef CAS.
- B. Duan, F. Liu, M. He and L. Zhang, Green Chem., 2014, 16, 2835 RSC.
- (a) S. Hajji, I. Younes, O. Ghorbel-Bellaaj, R. Hajji, M. Rinaudo, M. Nasri and K. Jellouli, Int. J. Biol. Macromol., 2014, 65, 298 CrossRef CAS PubMed; (b) E. Brunner, P. Richthammer, H. Ehrlich, S. Paasch, P. Simon, S. Ueberlein and K. H. Pe'e, Angew. Chem., Int. Ed., 2009, 48, 9724 CrossRef CAS PubMed; (c) H. Ehrlich, P. Simon, W. Carrillo-Cabrera, V. V. Bazhenov, J. P. Botting, M. Ilan, A. V. Ereskovsky, G. Muricy, H. Worch, A. Mensch, R. Born, A. Springer, K. Kummer, D. V. Vyalikh, S. L. Molodtsov, D. Kurek, M. Kammer, S. Paasch and E. Brunner, Chem. Mater., 2010, 22, 1462 CrossRef CAS; (d) H. Ehrlich, Int. Geol. Rev., 2010, 52, 661 CrossRef.
- (a) W. Ogasawara, W. Shenton, S. A. Davis and S. Mann, Chem. Mater., 2000, 12, 2835 CrossRef CAS; (b) F. A. Al Sagheer, M. A. Al-Sughayer, S. Muslim and M. Z. Elsabee, Carbohydr. Polym., 2009, 77, 410 CrossRef CAS.
- (a) A. M. tsuoka, T. Isogawa, Y. Morioka, B. R. Knappett, A. E. H. Wheatley, S. Saito and H. Naka, RSC Adv., 2015, 5, 12152 RSC; (b) V. Q. Nguyen, M. Ishihara, S. Nakamura, H. Hattori, T. Ono, Y. Miyahira and T. Matsui, J. Nanomater., 2013, 2013, 1 Search PubMed.
- H. Ehrlich, P. Simon, M. Motylenko, M. Wysokowski, V. V. Bazhenov, R. Galli, A. L. Stelling, D. Stawski, M. Ilan, H. Stocker, B. Abendroth, R. Born, T. Jesionowski, K. J. Kurzydłowskii and D. C. Meyer, J. Mater. Chem. B, 2013, 1, 5092 RSC.
- M. Wysokowski, I. Petrenko, A. L. Stelling, D. Stawski, T. Jesionowski and H. Ehrlich, Polymers, 2015, 7, 235 CrossRef CAS.
- M. Wysokowski, M. Motylenko, J. Walter, G. Lota, J. Wojciechowski, H. Stöcker, R. Galli, A. L. Stelling, C. Himcinschi, E. Niederschlag, E. Langer, V. V. Bazhenov, T. Szatkowski, J. Zdarta, I. Pertenko, Z. Kljajić, T. Leisegang, S. L. Molodtsov, D. C. Meyer, T. Jesionowski and H. Ehrlich, RSC Adv., 2014, 4, 61743 RSC.
- M. Wysokowski, M. Motylenko, J. Beyer, A. Makarova, H. Stöcker, J. Walter, R. Galli, S. Kaiser, D. Vyalikh, V. V. Bazhenov, I. Petrenko, A. L. Stelling, S. L. Molodtsov, D. Stawski, K. J. Kurzydłowski, E. Langer, M. V. Tsurkan, T. Jesionowski, J. Heitmann, D. C. Meyer and H. Ehrlich, Nano Res., 2015, 8, 2288 CrossRef CAS.
- M. Wysokowski, M. Motylenko, V. V. Bazhenov, D. Stawski, I. Petrenko, A. Ehrlich, T. Behm, Z. Kljajic, A. L. Stelling, T. Jesionowski and H. Ehrlich, Front. Mater. Sci., 2013, 7, 248 CrossRef.
- (a) A. R. Katritzky, B. V. Rogovoy and K. V. Kovalenko, J. Org. Chem., 2003, 68, 4941 CrossRef CAS PubMed; (b) M. L. Kantam, K. B. S. Kumar and K. P. Raja, J. Mol. Catal. A: Chem., 2006, 247, 186 CrossRef CAS.
- (a) S. J. Wittenberger, Recent development in tetrazole chemistry: a review, Org. Prep. Proced. Int., 1994, 26, 499 CrossRef CAS; (b) N. P. Gaponik, S. V. Voitekhovich and O. A. Ivashkevich, Russ. Chem. Rev., 2006, 75, 507 CrossRef.
- (a) R. J. Herr, Bioorg. Med. Chem., 2002, 10, 3379 CrossRef CAS PubMed; (b) L. V. Myznikov, A. Hrabalek and G. I. Koldobskii, Chem. Heterocycl. Compd., 2007, 43, 1 CrossRef CAS; (c) J. Roh, K. Vávrová and A. Hrabálek, Eur. J. Org. Chem., 2012, 2012, 6101 CrossRef CAS; (d) G. I. Koldobskii, Russ. J. Org. Chem., 2006, 42, 487 CrossRef.
- (a) R. N. Butler, in Comprehensive Heterocyclic Chemistry II, ed. A. R. Katritzky, C. W. Rees and E. F. V. Scriven, Pergamon Press, Oxford, 1996, vol. 4, p. 674 Search PubMed; (b) R. N. Butler, in Comprehensive Heterocyclic Chemistry II, ed. A. R. Katritzky and C. W. Rees, Pergamon Press, Oxford, 1984, vol. 5, p. 791 Search PubMed.
- D. Cantillo, B. Gutmann and C. O. Kappe, J. Org. Chem., 2012, 77, 10882 CrossRef CAS PubMed.
- A. Teimouri and A. Najafi Chermahini, Polyhedron, 2011, 30, 2606 CrossRef CAS.
- D. Kong, Y. Liu, J. Zhang, H. Li, X. Wang, G. Liu, B. Li and Z. Xu, New J. Chem., 2014, 38, 3078 RSC.
- M. Rahman, A. Roy, M. Ghosh, S. Mitra, A. Majee and A. Hajra, RSC Adv., 2014, 4, 6116 RSC.
- D. Habibi, M. Nasrollahzadeha and T. A. Kamali, Green Chem., 2011, 13, 3499 RSC.
- U. Yapuri, S. Palle, O. Gudaparthi, S. R. Narahari, D. K. Rawat, K. Mukkanti and J. Vantikommu, Tetrahedron Lett., 2013, 54, 4732 CrossRef CAS.
- B. Akhlaghinia and S. Rezazadeh, J. Braz. Chem. Soc., 2012, 23, 2197 CrossRef CAS.
- N. Razavi and B. Akhlaghinia, RSC Adv., 2015, 5, 12372 RSC.
- S. S. E. Ghodsinia and B. Akhlaghinia, RSC Adv., 2015, 5, 49849 RSC.
- R. Jahanshahi and B. Akhlaghinia, RSC Adv., 2015, 5, 104087 RSC.
- P. N. Tshibangu, S. N. Ndwandwe and E. D. Dikio, Int. J. Electrochem. Sci., 2011, 6, 2201 CAS.
- H. Valizadeh, M. Amiri and E. Khalili, Mol. Diversity, 2012, 16, 319 CrossRef CAS PubMed.
- (a) K. Skołucka-Szary, A. Ramięga, W. Piaskowska, B. Janicki, M. Grala, P. Rieske, Z. Bartczak and S. Piaskowski, Mater. Sci. Eng., C, 2016, 60, 489 CrossRef PubMed; (b) H. Ehrlich, M. Krautter, T. Hanke, P. Simon, C. Knieb, S. Heinemann and H. Worch, J. Exp. Zool., Part B, 2007, 308, 347 CrossRef PubMed.
- J. Zhou, Z. Dong, H. Yang, Z. Shi, X. Zhou and R. Li, Appl. Surf. Sci., 2013, 279, 360 CrossRef CAS.
- H. Tang, W. Zhou, A. Lu and L. Zhang, J. Mater. Sci., 2014, 49, 123 CrossRef CAS.
- M. Zarghani and B. Akhlaghinia, Appl. Organomet. Chem., 2015, 29, 683 CrossRef CAS.
- M. Wysokowski, M. Motylenko, H. Stocker, V. V. Bazhenov, E. Langer, A. Dobrowolska, K. Czaczyk, R. Galli, A. L. Stelling, T. Behm, Ł. Klapiszewski, D. A. zewicz, M. Nowacka, S. L. Molodtsov, B. Abendroth, D. C. Meyer, K. J. Kurzydłowski, T. Jesionowskia and H. Ehrlich, J. Mater. Chem. B, 2013, 1, 6469 RSC.
- (a) G. Marcelo, A. Muñoz-Bonilla, J. Rodríguez-Hernández and M. Fernández-García, Polym. Chem., 2013, 4, 558 RSC; (b) Z. Xu, C. Shen, Y. Tian, X. Shi and H. J. Gao, Nanoscale, 2010, 2, 1027 RSC.
- A. O. Dhokte, M. A. Sakhare, M. K. Lande and B. R. Arbad, J. Korean Chem. Soc., 2013, 57, 73 CrossRef CAS.
- D. R. Patil, Y. B. Wagh, P. G. Ingole, K. Singh and D. S. Dalal, New J. Chem., 2013, 37, 3261 RSC.
- F. Dehghani, A. R. Sardarian and M. Esmaeilpour, J. Organomet. Chem., 2013, 743, 87 CrossRef CAS.
- M. Abd El Aleem Ali Ali El-Remaily and A. M. Abu-Dief, Tetrahedron, 2015, 71, 2579 CrossRef.
- P. Mani, C. Sharma, S. Kumar and S. K. Awasthi, J. Mol. Catal. A: Chem., 2014, 392, 150 CrossRef CAS.
- P. Sivaguru, P. Theerthagiri and A. Lalitha, Tetrahedron Lett., 2015, 56, 2203 CrossRef CAS.
- P. Sivaguru, K. Bhuvaneswari, R. Ramkumar and A. Lalitha, Tetrahedron Lett., 2014, 55, 5683 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra07252f |
| This journal is © The Royal Society of Chemistry 2016 |