Environmentally friendly cyclotrimerization of substituted acetophenones catalyzed by a new nanocomposite of γ-Al2O3 nanoparticles decorated with H5PW10V2O40†
Reza Tayebee*a,
Kokab Savojib,
Maryam Kargar Razib and
Behrooz Malekia
aDepartment of Chemistry, Hakim Sabzevari University, Sabzevar, Iran. E-mail: Rtayebee@hsu.ac.ir; Fax: +98-51-44410300; Tel: +98-51-44410310
bDepartment of Chemistry, Islamic Azad University of Tehran North Branch, Tehran, Iran
First published on 25th May 2016
Abstract
A highly efficient and simple procedure for the synthesis of substituted benzenes is described. A range of aryl and alkyl ketones were used to conduct triple condensation reactions in the presence of a catalytic amount of nano-Al2O3/H5PW10V2O40 as an efficient and recyclable catalyst under solvent-free conditions. The supported heterogeneous catalyst was characterized using SEM, XRD, DLS, UV-Vis and FT-IR spectroscopy. All condensation reactions were completed in a suitable time compared to existing methods without formation of any undesirable 1,2,4-trisubstituted by-products. The entire synthetic sequence is cost-effective, environmentally benign, and possesses convenient generality, which makes the method meritorious as a valid contribution to the existing methods in the field of highly substituted benzene synthesis.
1. Introduction
Screening new efficient, high yielding, and environmentally benign synthetic transformations based on the principles of green chemistry and sustainability has been one of the major challenges of the past decades.1–4 Moreover, in the context of extending green synthetic strategies, solvent-free conditions are also a promising and essential facet of green chemistry through diminishing the consumption of hazardous organic solvents in a scaled-down reaction and achieving energy savings.Polyarylated benzene propellers have attracted a great deal of enthusiasm in cutting edge carbon nanotechnology, developing new efficient molecular rotors, and production of new electroluminescent materials for flat panel displays.5 1,3,5-Triarylbenzenes are important polycyclic aromatic compounds displaying potential applications in chemical industries such as nanomaterials,6 photovoltaic resisting materials,7 conducting polymers,8 electroluminescent devices,9 and various conjugated polyaromatics.10 A number of synthetic strategies have been developed previously to construct 1,3,5-triarylbenzenes. The most common methods include the metal catalyzed cyclotrimerization of alkynes,11–14 cross coupling reactions of aryl halides,15–18 and triple self-condensation of aryl methyl ketones in the presence of an acidic catalyst such as HCl,19 SiCl4,20 TiCl4,21 para-toluenesulfonic acid,22 Bi(OTf)3·4H2O,23 zirconocenebis(perfluoroctanesulfonate)s,24 ethylenediamine or trifluoroacetic acid.25 Condensation of aryl acetones mediated by Lewis acids provides an alternative pathway which only supplies C3-symmetrical 1,3,5-triarylbenzenes. However, in spite of their potential utility, these methods typically suffer from one or more disadvantages such as problematic substrates, use of a stoichiometric amount of catalyst, production of excessive waste, moisture sensitivity, specialized handling requirements, tedious workup, lack of generality, requirement of a transition metal as well as complicated ligands, and non-recyclability of the catalyst. Therefore, investigation of green routes by using renewable catalytic systems to synthesize 1,3,5-triarylbenzenes is highly desirable.
Heteropolyacids are strong and environmentally benign acid catalysts which contain sufficient acidic sites and have been explored as prominent catalysts in organic transformations.26–28 These inorganic metal–oxygen clusters bear high dispersion of negative charge over many atoms of the polyanion by exploiting strong Brønsted acid sites over the outer surface of the polyanion. However, some of their inherent drawbacks such as high solubility in aqueous solution and continuous leakage during operation limit the scope of their practical applications. To overcome these restrictions, heterogenization of the heteropolyacid through immobilization onto different weakly acidic or non-basic carriers is recommended. Up to now, several solid acids such as zeolites,27,28 MCM-41,29 MCM-48,30 and SBA-15 (ref. 31) have been reported to support heteropolyacids via immobilization.
γ-Al2O3 is perhaps one of the most important nanomaterials employed as support for metal catalysts and is considered as a promising material for a variety of applications due to its distinctive chemical, mechanical, and thermal properties.32 Therefore, γ-Al2O3 could be a good candidate to accommodate and deposit heteropolyacids and produce effective catalysts in organic synthesis by consideration of either Lewis and/or Brønsted acidic characteristics.32
To continue our efforts in the development of one-pot syntheses33–35 for various biologically important compounds and owing to the importance of 1,3,5-triarylbenzenes, herein an effective route for the preparation of these compounds is disclosed via the triple self-condensation of aryl methyl ketones in the presence of a new heterogeneous catalyst composed of nano-γ-Al2O3 and H5PW10V2O40 (HPA) under solvent-free conditions (Scheme 1). The present solvent-free methodology is effective and provides only the desired C3-symmetrical 1,3,5-triarylbenzenes in high yields by employing a simple, economic, and reusable heterogeneous catalyst without producing waste.
2. Experimental
2.1. Materials and methods
All reagents and starting materials were commercially available and used as received. Hydrophilic γ-aluminum oxide nanopowder (>99%) was purchased from NanoSany Corporation, Iran. The progress of reactions was monitored by TLC on silica gel polygram SIL G/UV 254 plates. Melting points were recorded on a Bamstead Electrothermal type 9200 melting point apparatus. Scanning electron microscope (SEM) micrographs and energy-dispersive X-ray (EDX) analysis were obtained using a LEO 440i microscope (acceleration voltage 26 kV). Transmission electron microscopy (TEM) images were obtained using a Philips Zeiss-EM10C transmission electron microscope with an accelerating voltage of 80 kV. X-ray diffraction patterns were obtained on a PW1840 (PHILIPS) diffractometer with Cu Kα radiation at 40 keV and 30 mA with a scanning rate of 3° min−1 in a 2θ range from 1° to 80°. Hydrodynamic particle size analysis of nano-γ-Al2O3/H5PW10V2O40 in an ethanol suspension was determined by measuring dynamic light scattering using a Zeta Sizer-HT (Brookhaven). Fourier transform infrared (FT-IR) spectra were recorded on an 8700 Shimadzu Fourier Transform spectrophotometer in the region of 400 to 4000 cm−1 using KBr pellets. Ultraviolet-visible spectra were recorded on a Photonix UV-Vis Array spectrophotometer, Model Ar 2015, Iran. The tungsten and vanadium content of the nanocatalyst was determined using Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) conducted on a Varian Vista-Pro model ICP-MS spectrometer. A freeze dryer, Model FD-10, Pishtaz Equipment Engineering Co, Iran, was used for drying the prepared nanocomposites. 1H and 13C NMR spectra were recorded on a Bruker AVANCE instrument operated at 300 MHz using TMS as an internal reference. All products were identified by comparison of their spectral and physical data with those previously reported.36 H5PW10V2O40·30H2O was prepared according to reported procedures.372.2. Surface modification of γ-alumina nanoparticles with different modifiers
Surface modification was performed by adding 0.9 g of nano-alumina to 0.1 g of the modifier dissolved in 5 ml of methanol. Then, the resulting solution was stirred for 6 h at room temperature. Finally, the mixture was heated gently to 40 °C until a white solid material was achieved. The resulting composites were dried at 80 °C for 8 h.2.3. Preparation of γ-Al2O3/H5PW10V2O40 nanocomposite
H5PW10V2O40 was supported on γ-Al2O3 nanoparticles via wet impregnation method. In this procedure, 0.9 g of nano-alumina was dispersed in 5 ml of a methanolic solution of HPA (0.1 g/5 ml). This mixture was stirred at room temperature for 6 h, which was supposed to be long enough to attain equilibrium of the adsorption–desorption processes. The resultant mixture was dried slowly at 40 °C until a white solid material was attained. Then, the sample was kept at 80 °C for 8 h for complete drying. To study effect of drying conditions on the catalytic efficacy of the nanocomposite, a freeze drying technique was compared with the usual classic drying in an oven at 80 °C under air. The results obtained demonstrate no obvious improvement in catalyst efficiency under the freeze drying conditions.2.4. General procedure for the cyclotrimerization of acetophenones into 1,3,5-triphenylbenzenes
In a typical reaction, acetophenone (1 mmol) and γ-Al2O3/HPA (0.02 g) were added to a small test tube and the reaction mixture was stirred at 90 °C for the required time. After completion of the reaction, as indicated by TLC using n-hexane/ethyl acetate 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as an eluent, the reaction mixture was cooled to 25 °C, then hot ethanol was added and the mixture was stirred for 5 min. The insoluble catalyst was isolated via simple filtration. The filtrate containing water, as the only by-product of the cyclotrimerization, was concentrated under reduced pressure and finally the obtained crude product was purified through re-crystallization in EtOH
1 as an eluent, the reaction mixture was cooled to 25 °C, then hot ethanol was added and the mixture was stirred for 5 min. The insoluble catalyst was isolated via simple filtration. The filtrate containing water, as the only by-product of the cyclotrimerization, was concentrated under reduced pressure and finally the obtained crude product was purified through re-crystallization in EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (3
H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as confirmed by an intense single spot in TLC. The pure products were specified based on the spectral data and determination of their melting points.36
1) as confirmed by an intense single spot in TLC. The pure products were specified based on the spectral data and determination of their melting points.36
2.5. Calculation of pHpzc (point of zero charge pH)
The pHpzc is a point at which the surface acidic or basic functional groups no longer affect the pH value of the solution. The effect of surface modification with HPA on the acidity of γ-Al2O3 was studied by calculating pHpzc for both γ-Al2O3/HPA and γ-Al2O3 nanoparticles. The pH of a series of NaCl solutions (50 ml, 0.01 M) was adjusted to a value between 2 and 12 by the addition of HCl (0.1 M) or NaOH (0.1 M) solutions in closed Erlenmeyer flasks. Then, pH of the solutions was defined as the initial pH (pHI). Thereafter, 0.2 g of the modified γ-Al2O3/HPA (or unmodified γ-Al2O3) was added and the final pH (pHF) was measured after 48 h. Finally, the values of pHF vs. pHI and also pHI vs. pHI were plotted and the intersect of the lines provided the pHpzc.382.6. Representative spectral data of 1,3,5-triarylbenzenes
3. Results and discussion
3.1. Characterization and physicochemical properties of γ-Al2O3/HPA nanoparticles
The physicochemical and morphological properties of the supported γ-Al2O3/H5PW10V2O40 catalyst were investigated by means of FT-IR, UV-Vis, SEM, XRD, and DLS. The most informative technique for the investigation of the surface interactions between H5PW10V2O40 and nano-γ-Al2O3 is FT-IR. The spectra of free H5PW10V2O40 (A), HPA impregnated nano-γ-Al2O3 (B), and nano-γ-Al2O3 (C) were recorded for structural characterization purposes (Fig. S1†). The Keggin heteropolyacid shows four characteristic bands in the range of 750–1100 cm−1.39 The observed bands at around 778–785 and 850–890 cm−1 are assigned to W–O–W. The peaks about 950–998 and 1070–1090 cm−1 are associated with M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and P–O bonds, respectively, which distinctively indicate the neat structure of the Keggin framework for the heteropolyacid. In the FT-IR spectrum of γ-Al2O3/H5PW10V2O40, these four peaks slightly overlap with those of γ-Al2O3. However, spectrum (B) confirms that loading of HPA occurred and H5PW10V2O40 was successfully immobilized on the pores of the nano-γ-Al2O3 support.
O and P–O bonds, respectively, which distinctively indicate the neat structure of the Keggin framework for the heteropolyacid. In the FT-IR spectrum of γ-Al2O3/H5PW10V2O40, these four peaks slightly overlap with those of γ-Al2O3. However, spectrum (B) confirms that loading of HPA occurred and H5PW10V2O40 was successfully immobilized on the pores of the nano-γ-Al2O3 support.
The morphology of the γ-Al2O3/H5PW10V2O40 nanocatalyst was investigated using SEM and TEM microscopy (Fig. 1). The TEM and SEM images show that the mean particle size of the catalyst was clearly <40 nm. According to the TEM micrograph, the two observed morphologies are due to γ-Al2O3 (rod-like) and H5PW10V2O40 (sheet-like) nanoparticles (Fig. 1c). Actually, by introducing HPA into γ-Al2O3, no significant effect on the homogeneity of the material occurred and the structure of the nano-alumina was maintained. Concerning the chemical composition of γ-Al2O3/H5PW10V2O40, energy dispersive X-ray (EDX) analysis was obtained (Fig. S2†). The analysis of EDX spectrum proves the existence of tungsten, vanadium, oxygen, and aluminium in the nanocatalyst; therefore, immobilization of the heteropolyacid onto the nano γ-Al2O3 is justified.
 | ||
| Fig. 1 SEM micrograph of γ-Al2O3 (a), and SEM (b) and TEM (c) images of γ-Al2O3/H5PW10V2O40. (a) is reproduced with permission.48 | ||
Fig. 2 displays wide-angle XRD patterns of the pure heteropolyacid (a), bare γ-Al2O3 (b) and heteropolyacid loaded γ-Al2O3/HPA (c). The unmodified γ-Al2O3 exhibits peaks at 37.5, 39.6, 45.7 and 67.5° which are attributed to the typical pattern of γ-alumina (Fig. 2b). These peaks are also evident for the modified γ-Al2O3/HPA (Fig. 2c). However, most of the peaks corresponding to the heteropolyacid are absent or are strongly weakened in the XRD pattern of γ-Al2O3/HPA. These findings indicate that HPA clusters were too small or were successfully finely dispersed onto the γ-alumina surface rather than existing as the free crystalline solid acid.40
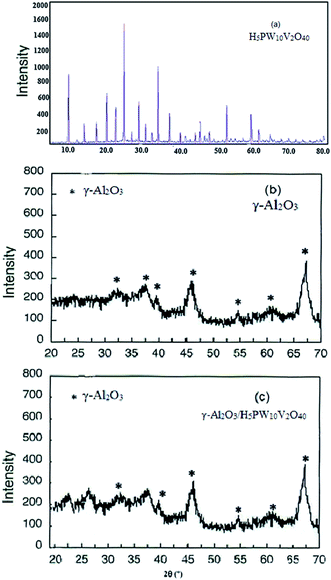 | ||
| Fig. 2 X-ray diffraction patterns of pure H5PW10V2O40 (a), γ-Al2O3 (b), and γ-Al2O3/H5PW10V2O40 (c) nanoparticles. | ||
The UV-Vis spectrum of HPA in methanol displays an absorption peak at 236 nm, which is assigned to the charge-transfer absorption in the heteropoly cage (Fig. 3). To find the extent of H5PW10V2O40 that can be loaded on the support, UV-Vis spectra of solutions containing HPA before and after addition of γ-alumina were obtained. For this purpose, 0.1 g of γ-Al2O3 was suspended in a 40 ml methanol solution of HPA with an initial concentration of 2500 ppm. The suspension was stirred for 120 min and absorption spectra were recorded for the solution after removing the solid catalyst by filtration (Fig. 3). Evidently, most of the heteropolyacid content was adsorbed on the alumina surface after 90 min. After stirring the solution and comparing with calibration curves for standard solutions, 0.026 mmol of H5PW10V2O40 was loaded per gram of the γ-Al2O3 support.
 | ||
| Fig. 3 Stacked plot of UV-Vis spectral changes with time after the addition of H5PW10V2O40 to γ-Al2O3. | ||
For confirmation of HPA loading on the support, 0.01 g of the heterogeneous nanocatalyst Al2O3/H5PW10V2O40 was analyzed by ICP-MS. The results indicate the presence of 0.22 μmol H5PW10V2O40. This finding is in good agreement with the results from UV-Vis analysis.
Finally, hydrodynamic particle size analysis of γ-Al2O3/H5PW10V2O40 was performed by measuring the dynamic light scattering of the nanoparticles in ethanol (Fig. S3†). The particle size histogram of the nanocatalyst shows that most of the particles range in size from 25–60 nm and possess an average size of 30 nm.
3.2. Equilibrium studies and adsorption isotherms
The equilibrium relationships between γ-Al2O3, as the adsorbent, and H5PW10V2O40, as the adsorbate, and the adsorption behavior of the heteropolyacid could be described by an adsorption isotherm. The shape of the isotherm not only provides information on the adsorption affinity of HPA, but also provides relevant information about adsorption spontaneity and the stability of adsorbent–adsorbate interactions.The amount of the Keggin heteropolyacid adsorbed onto γ-Al2O3 nanoparticles was calculated by studying the adsorption isotherm based on Freundlich and Langmuir patterns and the experimental data were fitted linearly.41,42 The Langmuir isotherm theory assumes monolayer coverage of the adsorbate over a homogenous adsorbent surface leading to surface homogeneity of the adsorbent; whereas the Freundlich adsorption isotherm is an indication of the surface heterogeneity of the adsorbent. According to the Langmuir adsorption model, the maximum adsorption would be due to the saturated monolayer of solute molecules on the adsorbent surface. The non-linear expression of the Langmuir model is given by eqn (1):
| q = abCe/(1 + bCe) | (1) |
3.3. Calculating the pHpzc of γ-Al2O3
Point of zero charge (pHpzc) is the value at which the surface of a solid inorganic material bears a net neutral charge. At pHpzc, the surface acidic (or basic) functional groups no longer contribute to the pH value of the solution.43 It encompasses positive charge in solution with pH < pHpzc; whereas, in solution with pH > pHpzc negative charge would be developed at the surface of the mineral compound. Therefore, changes to the protonation–deprotonation characteristics of Al–OH groups in the framework of γ-Al2O3 as a result of HPA loading caused some structural transformation which altered the pHpzc and therefore the equilibrium pH of the suspension.44 The pHpzc for γ-Al2O3 was analyzed to evaluate the effect of HPA loading on the acidity of the nano γ-alumina surface. The lower pHpzc for the modified γ-Al2O3/HPA (pHpzc = 5.7) compared to unmodified γ-Al2O3 confirms an increment in the acidity of nano-alumina after surface modification with the heteropolyacid (Fig. S5†).3.4. Catalytic tests
| Entry | Modifier | Nanocomposite | Yield% |
|---|---|---|---|
| a Reaction conditions: 1 mmol of acetophenone was mixed with 0.02 g of the nanocatalyst under solvent free conditions at 90 °C for 2.5 h.b 0.45 μmol of the heteropolyacids were used. Note that the mean value of 0.024 mmol HPA is considered per 1.07 g of the nanocatalyst. | |||
| 1 | — | γ-Al2O3 | 35 |
| 2 | H3PW12O40 | H3PW12O40/Al2O3 | 75 |
| 3 | H5PW10V2O40 | H5PW10V2O40/Al2O3 | 85 |
| 4 | CeO2 | CeO2/Al2O3 | 70 |
| 5 | H3PW12O40b | — | <3 |
| 6 | H5PW10V2O40b | — | <3 |
| Entry | Nanocatalyst (mg) | HPA (μmol) content | Yield (%) | |
|---|---|---|---|---|
| γ-Al2O3 (nano) | γ-Al2O3/HPA (nano) | |||
| a Reaction conditions: 1 mmol of acetophenone was mixed with the desired amount of catalyst under solvent-free conditions at 90 °C for 2.5 h. | ||||
| 1 | — | — | n. d. | n. d. |
| 2 | 5 | 0.11 | — | 50 |
| 3 | 10 | 0.22 | 15 | 63 |
| 4 | 15 | 0.33 | 22 | 77 |
| 5 | 20 | 0.44 | 35 | 85 |
| 6 | 30 | 0.66 | 38 | 85 |
| Entry | R | Time (h) | Yield (%) | Mp (°C) | Product | Ref. |
|---|---|---|---|---|---|---|
| a Reaction conditions: substrate (1 mmol), 90 °C, 0.02 g of γ-Al2O3/H5PW10V2O40, solvent-free conditions. All products were known and were identified by comparison of their spectral and physical data with those previously reported. | ||||||
| 1 | H | 2.5 | 85 | 172–174 | (Ph)3C6H3 | 36d |
| 2 | 4-OMe | 2.5 | 60 | 141–143 | (4-OMePh)3C6H3 | 36d |
| 3 | 4-Br | 2 | 85 | 263–264 | (4-BrPh)3C6H3 | 23 |
| 4 | 4-NO2 | 2.5 | 68 | 151–152 | (4-NO2Ph)3C6H3 | 36a |
| 5 | 4-Me | 3 | 82 | 177–179 | (4-MePh)3C6H3 | 23 |
| 6 | 4-OH | 3.5 | 77 | 237–239 | (4-OHPh)3C6H3 | 36a |
| 7 | 4-Cl | 2.5 | 93 | 247–249 | (4-ClPh)3C6H3 | 36d |
| Entry | Catalyst | Catalyst (mol%) | Solvent | Time (h) | Temp. (°C) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| a DBSA: 4-dodecylbenzenesulfonic acid. | |||||||
| 1 | H3PMo12O40 | 5 | Ethanol | 5 | Reflux | 87 | 21 |
| 2 | SnCl4 | 10 | Ethanol | 24 | Reflux | 55 | 45 |
| 3 | CuCl2 | 8 | Free | 4 | 155 | 76 | 46 |
| 4 | DBSAa | 20 | Free | 3 | 130 | 60 | 47 |
| 5 | HCl | 10 | Ethanol | 11 | Reflux | 45 | 46 |
| 6 | Amberlyst 15 | 30 | Toluene | 10 | Reflux | 60 | 36b |
| 7 | γ-Al2O3/HPA | 0.02 g | Free | 2.5 | 90 | 85 | This work |
γ-Al2O3 nanoparticles with a relatively high specific surface of >138 m2 g−1 have two types of Lewis and Brönsted acidic sites.48 The catalytic activity of the nanoparticles would be associated with the improved acidic properties of the surface after modification with the heteropolyacid. Lewis-type acid sites of alumina are formed during the dehydration process by combination of the two surface hydroxyl groups.49 Considering previous work,50 it is assumed that the heteropolyacid is physisorbed onto the surface of γ-Al2O3. However, strong Brönsted acid sites may be formed by the reaction between H5PW10V2O40 and the hydroxyl groups present on the surface of γ-Al2O3. Therefore, the approach of modifying a γ-Al2O3 surface with HPA can increase the concentration of the surface acidic sites.51,52
4. Conclusions
In conclusion, γ-Al2O3/H5PW10V2O40 is recommended as a useful, efficient, and recyclable heterogeneous catalyst for the cyclotrimerization of substituted aacetophenones to afford only C3-symmetrical 1,3,5-triarylbenzenes in high yields. The presented simple protocol shows several advantages over some reported literature methods from economic and environmental points of view, such as operational simplicity, no need to be protected from the atmosphere, short reaction time, mild reaction conditions, and good yields. Moreover, water was the only by-product of this reaction and the used catalyst was recyclable. This safe and clean procedure would be an acceptable candidate for automated applications.Acknowledgements
Partial financial support from the Research Councils of Hakim Sabzevari University and Islamic Azad University of Tehran, North Branch is greatly appreciated.References
- P. T. Anastas and T. C. Williamson, Green Chemistry: Frontiers in Benign Chemical Syntheses and Processes, Oxford Science Publications, New York, 1998 Search PubMed.
- A. S. Matlack, Introduction to Green Chemistry, Marcel Dekker, Inc., New York, 2001 Search PubMed.
- P. Tundo, A. Perosa and F. Zecchini, Methods and Reagents for Green Chemistry, John Wiley and Sons, Oxford, UK, 2007 Search PubMed.
- K. Tanaka and F. Toda, Chem. Rev., 2000, 100, 1025 CrossRef CAS PubMed.
- R. H. Friend, R. W. Gymer, A. B. Holmes, J. H. Burroughes, R. N. Marks, C. Taliani, D. D. C. Bradley, D. A. Dos Santos, I. L. Bredas, M. Logdlung and W. R. Salaneck, Nature, 1999, 397, 121 CrossRef CAS.
- T. Kadota, H. Kayeyama, F. Wakaya, K. Gamo and Y. Shirota, Chem. Lett., 2004, 33, 06 CrossRef.
- C. Li, M. Liu, N. G. Pschirer, M. Baumgarten and K. Müllen, Chem. Rev., 2010, 110, 6817 CrossRef CAS PubMed.
- R. Ghanbaripour, I. Mohammad poor-Baltork, M. Moghadam, A. R. Khosropour, S. Tangestaninejad and V. Mirkhani, J. Iran. Chem. Soc., 2012, 9, 791 CrossRef CAS.
- M. J. Plater, M. Mckay and T. Jackson, J. Chem. Soc., Perkin Trans. 1, 2000, 2695 RSC.
- (a) B. P. Dash, R. Satapathy, E. R. Gaillard, J. A. Maguire and N. S. Hosmane, J. Am. Chem. Soc., 2010, 132, 6578 CrossRef CAS PubMed; (b) Q. Wang, C. Zhang, B. C. Noll, H. Long, Y. Jin and W. Zhang, Angew. Chem., Int. Ed., 2014, 53, 10663 CrossRef CAS PubMed.
- K. Yoshida, I. Morimoto, K. Mitsudo and H. Tanaka, Tetrahedron, 2008, 64, 5800 CrossRef CAS.
- G. Hilt, C. Hengst and W. Hess, Eur. J. Org. Chem., 2008, 2293 CrossRef CAS.
- Y. Xu, Y. Pan, Q. Wu, H. Wang and P. Liu, J. Org. Chem., 2011, 76, 8472 CrossRef CAS PubMed.
- A. Ciammaichella, A. Leoni and P. Tagliatesta, New J. Chem., 2010, 34, 2122 RSC.
- S. Shi and Y. Zhang, J. Org. Chem., 2007, 72, 5927 CrossRef CAS PubMed.
- L. Bai and J. X. Wang, Adv. Synth. Catal., 2008, 350, 315 CrossRef CAS.
- M. Mora, C. Jimenez-Sanchidrian and J. R. Ruiz, Appl. Organomet. Chem., 2008, 22, 122 CrossRef CAS.
- S. Paul and J. H. Clark, Green Chem., 2003, 5, 635 RSC.
- R. E. Lyle, E. J. DeWitt, N. M. Nichols and W. Cleland, J. Am. Chem. Soc., 1953, 75, 5959 CrossRef CAS.
- M. J. Plater and M. Praveen, Tetrahedron Lett., 1997, 38, 1081 CrossRef CAS.
- Z. Li, W. H. Sun, X. Jin and C. Shao, Synlett, 2001, 1947 CrossRef CAS.
- Y. Zhao, J. Li, C. Li, K. Yin, D. Ye and X. Jia, Green Chem., 2010, 12, 1370 RSC.
- F. Ono, Y. Ishikura, Y. Tada, M. Endo and T. Sato, Synlett, 2008, 15, 2365 Search PubMed.
- G. P. Zhang, R. H. Qiu, X. H. Xu, H. H. Zhou, Y. F. Kuang and S. H. Chen, Synth. Commun., 2012, 42, 858 CrossRef CAS.
- S. Zhang, Z. Xue, Y. Gao, S. Mao and Y. Wang, Tetrahedron Lett., 2012, 53, 2436 CrossRef CAS.
- R. Tayebee, N. Abdollahi and M. Ghadamgahi, J. Chin. Chem. Soc., 2013, 60, 1014 CrossRef CAS.
- R. Tayebee, M. M. Amini, F. Nehzat, O. Sadeghi and M. Armaghan, J. Mol. Catal. A: Chem., 2013, 366, 140 CrossRef CAS.
- R. Tayebee, M. M. Amini, N. Abdollahi, A. Aliakbari, S. Rabiei and H. Ramshini, Appl. Catal., A, 2013, 468, 75 CrossRef CAS.
- R. Tayebee, M. M. Amini, M. Akbari and A. Aliakbari, Dalton Trans., 2015, 44, 9596 RSC.
- R. Tayebee and B. Maleki, J. Chem. Sci., 2013, 125(2), 335 CrossRef CAS.
- R. Tayebee, M. M. Amini, M. Ghadamgahi and M. Armaghan, J. Mol. Catal. A: Chem., 2013, 366, 266 CrossRef CAS.
- (a) R. Rohman, H. Mecadon, A. T. Khan and B. Myrboh, Tetrahedron Lett., 2012, 53, 5261 CrossRef; (b) L. Cheng and X. P. Ye, Catal. Lett., 2009, 130, 100 CrossRef CAS; (c) A. Eliassi and M. Ranjbar, International Journal of NanoScience and Nanotechnology, 2014, 10, 13 Search PubMed; (d) B. F. Mirjalili, A. Bamoniri and S. M. Nezamalhosseini, J. Nanostruct. Chem., 2015, 5, 367 Search PubMed; (e) D. Salari, A. Niaei, S. A. Hosseini, R. Aleshzadeh and H. Afshary, J. Nanosci. Nanotechnol., 2010, 6, 23 Search PubMed; (f) D. R. Abd El-Hafiz, M. Riad and S. Mikhail, J. Nanostruct. Chem., 2015, 5, 393 CrossRef; (g) M. Salehi and E. Arabsarhangi, Int. Nano Lett., 2015, 5, 141 CrossRef CAS; (h) A. Badiei, M. Azmard, M. Karimi and P. Zarabadi-poor, J. Nanostruct. Chem., 2014, 4, 259 Search PubMed; (i) S. Sadjadi and S. Rasouli, Int. J. Nano Dimens., 2011, 1, 177 CAS; (j) S. V. Atghia and S. S. Beigbaghlou, J. Nanostruct. Chem., 2013, 3, 38 CrossRef.
- R. Tayebee, M. M. Amini, S. Pouyamanesh and A. Aliakbari, Dalton Trans., 2015, 44, 5888 RSC.
- R. Tayebee, B. Maleki, F. M. Zonoz, R. M. Kakhki and T. Kunani, RSC Adv., 2016, 6, 20687 RSC.
- R. Tayebee, M. M. Amini, H. Rostamian and A. Aliakbari, Dalton Trans., 2014, 43, 1550 RSC.
- (a) H. R. Safaei, M. Davoodi and M. Shekouhy, Synth. Commun., 2013, 43, 2178 CrossRef CAS; (b) A. Kumar, M. Dixit, S. P. Singh, A. Goel, R. Raghunandan and P. R. Maulik, Tetrahedron Lett., 2009, 50, 4335 CrossRef CAS; (c) X. Jing, F. Xu, Q. Zhu, X. Ren, C. Yan, L. Wang and J. Wang, Synth. Commun., 2005, 35, 3167 CrossRef CAS; (d) R. Ghanbaripour, I. Mohammadpoor-Baltork, M. Moghadam, A. R. Khosropour, S. Tangestaninejad and V. Mirkhani, Polyhedron, 2012, 31, 721 CrossRef CAS; (e) Q. Gao, F. Bao, X. Feng, Y. Pan, H. Wang and D. Li, ARKIVOC, 2013, 3, 49 Search PubMed.
- (a) E. R. Cadot, A. T. M. Thouvenot and G. Herve, Inorg. Chem., 1992, 31, 4128 CrossRef CAS; (b) G. A. Tsigdinos and C. J. Hallada, Inorg. Chem., 1968, 7, 437 CrossRef CAS.
- P. C. C. Faria, J. J. M. Orfao and M. F. R. Pereira, Water Res., 2004, 38, 2043 CrossRef CAS PubMed.
- (a) P. Karandikar, K. C. Dhanya and S. Deshpande, Catal. Commun., 2004, 69 CrossRef CAS; (b) R. Tayebee, F. Nehzat, E. Rezaei-Seresht, F. Z. Mohammadi and E. Rafiee, J. Mol. Catal. A: Chem., 2011, 351, 1837 CrossRef.
- (a) H. Cai, J. Mao, Y. Tao and X. Zheng, React. Kinet. Catal. Lett., 2009, 97, 43 CrossRef CAS; (b) M. G. Kulkarni, R. Gopinath, L. C. Meher and A. K. Dalai, Green Chem., 2006, 8, 1056 RSC.
- E. Korngold, N. Belayev and L. Aronov, Sep. Purif. Technol., 2003, 33, 179 CrossRef CAS.
- J. Febrianto, A. Kosasih, J. Sunarso, Y. Ju, N. Indraswati and S. Ismadji, J. Hazard. Mater., 2009, 162, 616 CrossRef CAS PubMed.
- O. K. Mahadwad, P. A. Parikh, R. V. Jasra and C. Patil, Bull. Mater. Sci., 2011, 34, 551 CrossRef CAS.
- H. Guan, E. Bestland, C. Zhu, H. Zhu, D. Albertsdottir, J. Hutson, C. T. Simmons, M. Ginic-Markovic, X. Tao and A. V. Ellis, J. Hazard. Mater., 2010, 183, 616 CrossRef CAS PubMed.
- K. Phatangare, V. Padalkar, D. Mhatre, K. Patil and A. Chaskar, Synth. Commun., 2009, 39, 4117 CrossRef CAS.
- N. O. Mahmoodi and N. Hajati, J. Chin. Chem. Soc., 2002, 49, 91 CrossRef CAS.
- D. Prasad, A. Preetam and M. Nath, C. R. Chim., 2013, 16, 252 CrossRef CAS.
- Iranian Nanomaterials Pioneers Nanosany Group, https://www.sites.google.com/site/nanosany.
- E. J. M. Hensen, D. G. Poduval, V. Degirmenci, D. A. J. M. Ligthart, W. Chen, F. Mauge, M. S. Rigutto and J. A. R. V. Veen, J. Phys. Chem. C, 2012, 116, 21416 CAS.
- R. Feng, S. Liu, P. Bai, K. Qiao, Y. Wang, H. A. Al-Megren, M. J. Rood and Z. Yan, J. Phys. Chem. C, 2014, 118, 6226 CAS.
- F. Dumeignil, M. Rigole, M. Guelton and J. Grimblot, Chem. Mater., 2005, 17, 2369 CrossRef CAS.
- S. A. El-Hakam and E. A. El-Sharkawy, Mater. Lett., 1998, 36, 167 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra07141d |
| This journal is © The Royal Society of Chemistry 2016 |