Purification, characterization and synthesis of antioxidant peptides from enzymatic hydrolysates of coconut (Cocos nucifera L.) cake protein isolates
Abstract
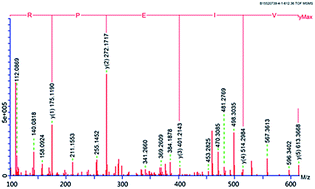
Papain was optimized to hydrolyze coconut cake protein isolates (CCPI) to produce peptides with high antioxidant activity. From the response surface methodology generated model, the optimum conditions were using papain at a concentration of 0.97 g/100 g CCPI, a hydrolysis time of 6.45 h, a temperature of 49.09 °C and a pH of 6.68. The coconut cake protein isolates hydrolysates were separated by G-25 gel chromatography and five fractions were obtained. The amino acid composition and antioxidant activity of the fractions were investigated. Fraction E with the highest antioxidant activity was further purified using gel filtration chromatography, ion exchange chromatography and RP-HPLC (reversed-phase high performance liquid chromatography). Finally, two peptides Pro-Gln-Phe-Tyr-Trp (865.02 Da) and Arg-Pro-Glu-Ile-Val (612.36 Da) were identified. Their IC50 (the concentration of peptide that is required to scavenge 50% of radical activity) on hydroxyl radical scavenging activities were 4.28 and 7.65 μg mL−1. Furthermore, these two peptides were chemically synthesized and the synthetic peptides showed good stability against simulated gastrointestinal protease digestion.

 Please wait while we load your content...
Please wait while we load your content...