Influence of the spacer on the phase behaviors of side-chain liquid crystalline copolymers based on triphenylene discotic mesogen unit
Jianfeng Ban*ab,
Luona Mua,
Lin Chend,
Shaojun Chen*a and
Hailiang Zhang*c
aShenzhen Key Laboratory of Special Functional Materials, College of Materials Science and Engineering, Shenzhen University, Shenzhen, 518060, Guangdong Province, China. E-mail: banban997@sina.com
bKey Laboratory of Optoelectronic Devices and Systems of Ministry of Education and Guangdong Province, College of Optoelectronic Engineering, Shenzhen University, Shenzhen, 518060, Guangdong Province, China
cKey Laboratory of Polymeric Materials and Application Technology of Hunan Province, Key Laboratory of Advanced Functional Polymer Materials of Colleges and Universities of Hunan Province, College of Chemistry, Xiangtan University, Xiangtan, 411105, Hunan Province, China
dSchool of Materials Science and Engineering, Baise University, Baise, 533000, People's Republic of China
First published on 1st April 2016
Abstract
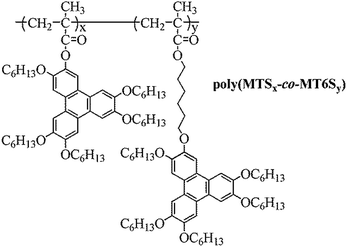
Based on two analogous triphenylene liquid crystalline monomers (MTS and MT6S), a novel series of binary copolymers poly{[3,6,7,10,11-pentakis(hexyloxy)-2-oxytriphenylene]methacrylate-co-6-[3,6,7,10,11-pentakis(hexyloxy)-2-oxytriphenylene]hexyl methacrylate} (poly(MTSx-co-MT6Sy)) were prepared by free radical polymerization using AIBN as initiator. The random nature of the copolymers was expected on the basis of the assumed similar reactivities due to the analogous triphenylene monomers. The phase behaviors of copolymers were studied by DSC, POM and 1D WAXD. The results showed that the content of PMT6S has a significant effect on the liquid crystalline (LC) phase behaviors and phase structures of copolymers. The comparison between PMTS and PMT6S indicates that the content of the spacer was crucial to determine the LC structures. The PMTS formed a stable columnar nematic phase (ΦN) and the PMT6S exhibited a stable hexagonal columnar phase (ΦH). After copolymerization, the glass transition temperature and the phase transition temperature of the copolymers from the LC phase to isotropic phase both decreased with the molar fraction of PMT6S in the feed. The samples whose molar content of PMT6S was below 75% formed stable ΦN, similar to the property of PMTS. The samples whose molar content of PMT6S was above 80% presented the symmetry ΦH, similar to the property of PMT6S. Through copolymerization, one can better understand the interrelation of microstructures and how Tp mesogenic orders constitute the key basis for various applications.
Introduction
Phase structures and transitions of thermotropic side-chain liquid crystalline polymers (SCLCPs) have been important topics to study in the chemistry and physics of polymers because SCLCPs have a great potential in functional soft materials.1–3 Therefore, many researchers have investigated the structure–property relationships of SCLCPs to understand the principles of structure formation and structure manipulation.4–9 As is well known, SCLCPs contain four distinct structural components: the backbone, the flexible spacer, the mesogenic group and the terminal substituent.10For mesogenic groups, because the discotic liquid crystals (DLCs) were first discovered by Chandrasekhar in 1977, DLC materials have attracted great attention due to their capability of self-assembly into well-ordered supramolecular structures because of the π–π stacking of the planar aromatic cores, which can be applied in organic semiconductors, superconducting materials, optical compensation films, one-dimensional conductors and photovoltaic solar cells.11–16 Among them, the phase behavior and phase structure of SCLCPs containing triphenylene (Tp) derivatives with long flexible spacers as linkers have been widely investigated, because of their relatively easy synthesis, thermal and chemical stability and variety of mesophases. Moreover, due to the “jacketing effect”, our group have investigated the structure–property relationships of SCLCPs containing Tp (PBTCS), to understand the principles of structure formation and structure manipulation.17 The results indicated that PBTCS has a relatively high glass transition temperature and formed a higher symmetry hexagonal columnar phase (ΦH) due to the strong coupling effect between the Tp and the MJLCP main chain.
The phase behavior of the SCLCPs can be tailored not only by changing chemical structure, but also the external conditions such as solvent, blends and copolymerization.18–23 Copolymerization represents the simplest synthetic technique that can be used to tailor the phase behaviors of the SCLCPs and to widen their application range as high-performance materials. For example, when 2,5-bis[(4-methoxyphenyl)oxycarbonyl]styrenes (MPCS) were copolymerized with non-liquid crystalline vinyl monomers, such as styrene (St) and methyl methacrylate (MMA), a mesophase can only be observed when the molar content of MPCS in copolymers exceeds about 89% and 84%, respectively.24 Tang25 has reported that 2,5-di(n-butoxycarbonyl)styrene was employed to tailor the mesomorphic property of PMPCS by random radical polymerization. Percec and Lee26,27 have systematically studied the influence of the length of the flexible spacer on the phase behavior of poly(x-[(4-cyano-40-biphenylyl)oxy]alkyl vinyl ethers and copolymers in which the mesogenic units were identical but the spacer lengths were varied.
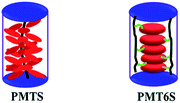
In our previous studies, we have systematically researched the influence of the spacer and molecular weight on the phase behavior of side-chain liquid crystalline polymers containing Tp discotic mesogen units as side groups (PMTS and PMT6S),28 and found that different length flexible spacers affect the self-organization of PMTnS.29 From our experimental results, the LC phase structures of PMTS were found to be strongly dependent on Mn, but PMT6S was independent on Mn. The length of spacer has a significant effect on the LC phase behavior of PMTnS. Moreover, we have proved that the PMTS formed the stable columnar nematic phase (ΦN), due to the bulky rigid side-chains of Tp wrapping around the main chain and the whole rigid columnar-shaped molecule of PMTS acting as a building block for the LC phase (see Scheme 1). The PMT6S exhibited the stable hexagonal columnar phase (ΦH) owing to the decoupling and self-organization of the Tp moieties (see Scheme 1).
In this study, it is necessary to understand the interrelation between Tp mesogenic orders and overall morphological structures, which is based on the acquisition of a series of well-defined Tp SCLCPs. Herein, we report the rational design and controllable synthesis of a novel series of Tp-based discotic copolymers (poly(MTSx-co-MT6Sy)) (see Scheme 2). We studied this copolymer based for two reasons. One is that the co-monomers used in this study were mesogenic analogues, which provide a convenient way to systematically investigate the effect of the spacer length difference on the mesomorphic properties of the copolymers. The other reason is that the phase behaviors of these two homopolymers have been systematically studied by our groups and show two different types of phase behavior due to the different interactions related to the presence, or not, of a long flexible spacer. Therefore, it would help us to better understand the mesomorphic properties of the copolymers. Through the study, we can obtain knowledge about the phase behavior and information on the structure variation of the copolymers. Studying the phase behavior and phase structures of these novel Tp copolymers will be useful for fundamental research and for real applications.
Experimental
Materials
Anhydrous tetrahydrofuran (THF) was distilled from sodium benzophenone ketyl under argon and used immediately. Triethylamine (TEA) and dichloromethane (CH2Cl2) were dried over anhydrous magnesium sulfate. 2,2-Azobisisobutyronitrile (AIBN) was freshly recrystallized from methanol. Chlorobenzene (Acros, 99%) was purified by washing with concentrated sulfuric acid to remove residual thiophenes, followed by two more washings, first with 5% sodium carbonate solution and then with water before it was dried with anhydrous calcium chloride and distilled. All other reagents and solvents were used as received without further purification.Instruments and measurements
All NMR measurements were performed on a Bruker ARX 400 MHz spectrometer using CDCl3 as solvent and tetramethylsilane (TMS) as the internal standard at ambient temperature. The chemical shifts were reported on the ppm scale.The apparent number average molecular weight (Mn) and polydispersity index (PDI = Mw/Mn) were measured on a GPC instrument (WATERS 1515) with a set of HT3, HT4 and HT5. The μ-styragel column used THF as eluent and the flow rate was 1.0 mL min−1 at 38 °C. The GPC data were calibrated with polystyrene standards.
TGA was performed on a TA SDT 2960 instrument at a heating rate of 20 °C min−1 in a nitrogen atmosphere.
DSC traces of the polymer were obtained on a TA Q10 DSC instrument. The temperature and heat flow were calibrated using standard materials (indium and zinc) at a cooling and heating rates of 10 °C min−1. The sample with a typical mass of about 5 mg was encapsulated in sealed aluminum pans.
LC texture of the polymer was examined under polarized optical microscopy (POM, Leica DM-LM-P) equipped with a Mettler Toledo hot stage (FP82HT).
One-dimensional wide-angle X-ray diffraction (1D WAXD) experiments were performed on a BRUKER AXS D8 Advance diffractometer with a 40 kV FL tube as the X-ray source (Cu-Kα) and the LYNXEYE_XE detector. Background scattering was recorded and subtracted from the sample patterns. The heating and cooling rates in the 1D WAXD experiments were 10 °C min−1.
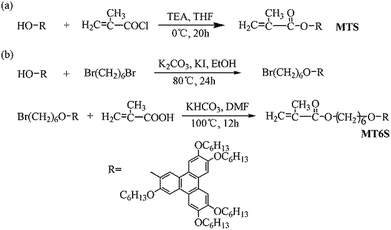
Synthesis of monomers
The precursor 2-hydroxyl-3,6,7,10,11-pentakis(hexyloxy)triphenylene (PHT) was synthesized according to previous study.17 The monomers, [3,6,7,10,11-pentakis(hexyloxy)-2-oxytriphenylene]methacrylate (MTS) and 6-[3,6,7,10,11-pentakis(hexyloxy)-2-oxytriphenylene]hexyl methacrylate (MT6S), were easy to synthesize and purified with reference to the procedures as described in our previous study.17 The synthetic procedures of the monomers are shown in Fig. 1. The chemical structures of the monomers were confirmed by 1H/13C NMR and mass spectrometry (MS).Synthesis of copolymers
As shown in Fig. 2, solution copolymerizations of MTS with MT6S, with different feed compositions, were carried out in chlorobenzene at 75 °C using AIBN as initiator (0.05%, based on the total molar quantities of monomers). For example, the polymerization procedures of P6 is described as follows: MTS (0.05 g, 0.06 mmol), MT6S (0.2 g, 0.22 mmol), 2 mL of chlorobenzene, the appropriate amount of AIBN and a magnetic stirrer bar were added into a polymerization tube. The tube was purged with nitrogen and subjected to three freeze–pump–thaw cycles to remove any dissolved oxygen, then sealed under vacuum. Polymerization was performed at 75 °C for 8 h with constant stirring. Subsequently, the polymerization was stopped by dipping the tube in ice water. The reaction mixture was then diluted with 10 mL of THF. The resultant polymer was precipitated and washed with 300 mL of methanol. To eliminate the unreacted monomers completely, the purification was repeated three times, until no peak was observed at the elution time of the monomer in the gel permeation chromatography (GPC) measurement. Following with drying of the copolymer at 50 °C under vacuum for 24 h, the target copolymer of poly(MTS20-co-MT6S80) (P6) was obtained.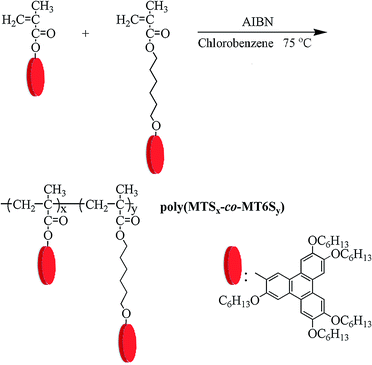 | ||
| Fig. 2 Synthesis and chemical structures of poly(MTSx-co-MT6Sy); x and y represent the molar ratios of the two structural units in the feed. | ||
Results and discussion
Synthesis and characterization of monomers and copolymers
The copolymerizations of MTS with MT6S in chlorobenzene were studied with different monomer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) monomer ratios in the feed. GPC analysis was performed to determine Mn and the molecular weight distributions of the copolymers. To exclude the effect of Mn on the LC behavior, all copolymers were obtained with a high enough Mn (Mn > 8.5 × 104 g mol−1). Table 1 summarizes the copolymerization results for the copolymers; we can also observe that there are nearly the same high Mn values for all samples. Due to the similarity in the structures of MTS and MT6S, the reactivity of the monomers was assumed to be equal. As can be observed from the result, the molar percentage of MT6S in the feed ranges from 0 to 1.
monomer ratios in the feed. GPC analysis was performed to determine Mn and the molecular weight distributions of the copolymers. To exclude the effect of Mn on the LC behavior, all copolymers were obtained with a high enough Mn (Mn > 8.5 × 104 g mol−1). Table 1 summarizes the copolymerization results for the copolymers; we can also observe that there are nearly the same high Mn values for all samples. Due to the similarity in the structures of MTS and MT6S, the reactivity of the monomers was assumed to be equal. As can be observed from the result, the molar percentage of MT6S in the feed ranges from 0 to 1.
| Samples | MTSx![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MT6Sya MT6Sya |
Mn (×104)b | PDIb | Tgc (°C) | Tid (°C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
a MTSx![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MT6Sy is the mole fraction of MTS to mole fraction of MT6S ratio in the feed.b Obtained from the Waters 1515 instrument, linear PS as standards.c Evaluated by DSC during the second heating process under an N2 atmosphere, at a rate of 10 °C min−1.d Evaluated by POM at a heating rate of 10 °C min−1. MT6Sy is the mole fraction of MTS to mole fraction of MT6S ratio in the feed.b Obtained from the Waters 1515 instrument, linear PS as standards.c Evaluated by DSC during the second heating process under an N2 atmosphere, at a rate of 10 °C min−1.d Evaluated by POM at a heating rate of 10 °C min−1. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| P1 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
8.9 | 1.83 | 158 | 233 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| P2 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
12.0 | 2.15 | 92 | 142 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| P3 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60 |
9.3 | 2.03 | 85 | 138 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| P4 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 70 |
11.3 | 2.23 | 71 | 133 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| P5 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 75 |
9.9 | 1.98 | 67 | 121 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| P6 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
8.9 | 2.40 | 56 | 117 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| P7 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 90 |
10.9 | 2.17 | 44 | 106 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| P8 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
8.5 | 1.82 | 42 | 86 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Phase transitions and phase structures of copolymers
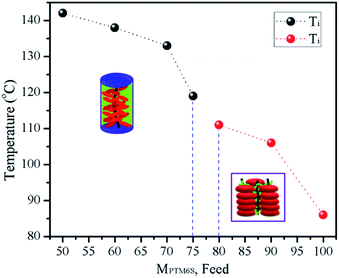
Thermal stability is an important property in potential applications. The TGA test results revealed that all copolymers showed good thermal stability, almost no weight loss at temperatures as high as 380 °C. The dependence of the thermal transition temperatures on the compositions of copolymers (poly(MTSx-co-MT6Sy)) were studied by DSC experiments. Fig. 3 shows the second heating DSC curves of copolymers at a rate of 10 °C min−1 under nitrogen atmosphere after eliminating the thermal history. In our previous studies, we had particularly researched the phase behaviors of PMTS and PMT6S. The experimental results showed that only the Tg can be detected for PMTS because the PMTS acted like many Mesogen jacket liquid crystal polymers (MJLCPs)31 main chain and Tp as a whole, while the Tg and Ti can be detected for PMT6S. The Ti of copolymers, which was determined by the POM investigation, are summarized in Table 1. As can be observed in the DSC curves (Fig. 3), each copolymer shows a single glass transition temperature; to see the Tg value clearly in Fig. 3, taking P7 and P6 as examples, the curve which belong to the Tg value are magnified ×3 and circled. The others can be observed in Fig. 3, to which the arrows are pointing. The single Tg indicates that the copolymers were homogeneous. From the transitional phenomenon of the copolymers, the copolymers could be divided into two types. The first type was P2–P5, whose molar content of PMT6S was below 75% and shows only a glass transition step with no endothermic peak correlating with a phase transition. This phenomenon is similar to PMTS. Moreover, the second type is P6 and P7, whose molar content of PMT6S is above 80%, as shown in Fig. 3 and a glass transition step and an endothermic peak are observed. Combining the POM and 1D WAXD experimental results, this endothermic peak was attributed to the transitions from the LC phase to an isotropic phase.30 Moreover, with the molar content of PMT6S increasing from P2 to P7, the Tg slightly decreases from 92 °C to 44 °C (see Table 1), indicating that the more flexible the spacer (MT6S) contained in the copolymers, the easier it is to form the more stable hexagonal columnar phase.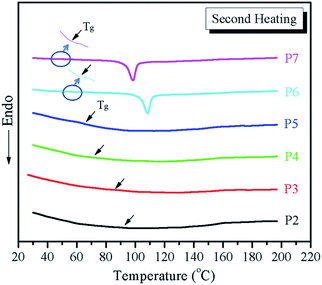 | ||
| Fig. 3 DSC curves of poly(MTSx-co-MT6Sy) during second heating scan at a rate of 10 °C min−1 under a N2 atmosphere. | ||
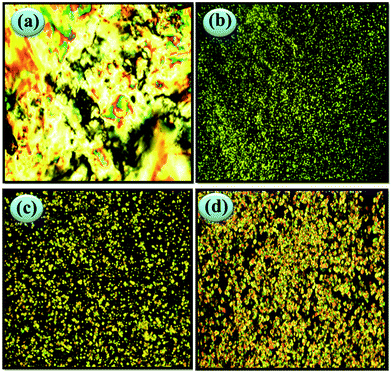
POM experiments further confirmed the DSC results. Thin films of the copolymers were prepared by a CH2Cl2 solution-cast method on clean cover glass, followed by slow drying at room temperature. To maintain consistency with the DSC results (second heating), the samples were heated to the same high temperature as the DSC test and then slowly cooled to room temperature. The POM experimental results also show that the copolymers could be divided into two groups. The first group was P2–P5, taking P2 (poly{MTS(0.50)-co-MT6S(0.50)}) as the example. As can be observed in Fig. 4a, P2 formed a colorful LC texture at room temperature. With the heating procedure, the samples maintained zero birefringence until over 142 °C. During cooling from 142 °C to room temperature, no LC textures were observed again. We considered the following mechanism to explain this phenomenon: when the molar content of PMT6S is below 75% the copolymers were acting as P1 (PMTS) forming a columnar nematic phase due to the strong coupling effect. To minimize the torque arising from the rigid side-chains of Tp, the backbone was compelled to stretch to a great extent, i.e., Tp would be wrapped around the main chain. The samples of P3, P4 and P5 showed similar results to P2.
The second group consisted of P6 and P7. As can be observed in Fig. 4b (P6) and Fig. 4c (P7), similar results to P8 (as can be observed in Fig. 4d) were obtained. With slow heating, the samples became soft and formed sand-like textures, suggesting the formation of the columnar phase. Once the temperature reached 117 °C (P6) and 106 °C (P7), the textures disappeared and the field of vision became dark, indicating that the copolymers entered into the isotropic state and a corresponding exothermic peak appeared in the DSC curve (Fig. 3). After cooling, the sand-like texture reappeared. We considered this phenomenon: as the molar content of PMT6S is above 80%, the copolymers were acting as P8 (PMT6S) forming hexagonal columnar phase, owing to the decoupling and self-organization of the Tp moieties. The transition temperatures from LC phase to isotropic phase (measured by POM) are listed in Table 1. As can be observed (Fig. 5), Ti and Tg decreased as the molar content of PMT6S increased, which agreed with the DSC result.
Phase structure identification of the polymer
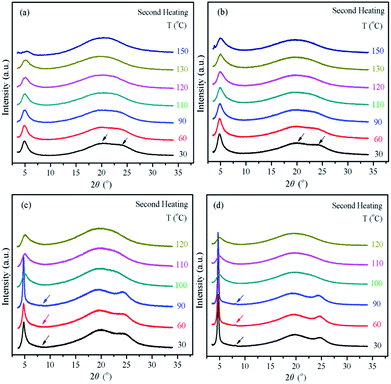
We carried out powder 1D WAXD experiments to collect more information concerning the molecular arrangements, mode of packing, and types of order in the mesophases of poly(MTSx-co-MT6Sy). To be consistent with the DSC and POM results, the samples were heated to 250 °C, and then slowly cooled to room temperature. The temperature was not higher than 300 °C during the experiments to avoid thermal degradation of the samples. Based on the 1D WAXD observations, the copolymers poly(MTSx-co-MT6Sy) could be divided into two groups, consistent with the POM and DSC results.In general, Tp DLCs tend to stack on one another to self-organize into a well-ordered supramolecular column because of the π–π stacking; this leads to many Tp derivatives, including Tp-based molecules' side-chain LC polymers, forming 2D ΦH or ΦN phases. The first group was P2–P5 and, to some extent, the results were similar to P1 (PMTS), which our group has proved formed the stable ΦN phase and acted just like many MJLCPS.31 The 1D WAXD powder patterns of P2 and P4 during the second heating step are shown in Fig. 6a and b, respectively. Taking P2 as the example, as shown in Fig. 6a, this renders only one peak in the low-angle region, 2θ = 4.92° (d = 1.79 nm), indicating the existence of an ordered structure, which can be identified as a ΦN phase developed by the main chain and Tp as a whole. Thus, we can say that this was a columnar ΦN phase. In the wide-angle region, at room temperature, two amorphous halos were found at 2θ values of ∼20° (d = 0.44 nm) and ∼24° (d = 0.37 nm), which represent the characteristic dimensions of amorphous packing of alkyl chains and the π–π stacking in a discotic LC column.32–34 The samples for P3, P4 and P5 showed similar results to P2. The 1D WAXD patterns for copolymers were used to calculate the diameter of the column and the results are listed in Table 2. As can be observed in Table 2, with the molar content of PMT6S increased, the diameter of the column increased from 1.78 nm to 1.85 nm. We speculate that with the content of spacer increased, the activity of Tp in the side-chain is increased, thus the copolymers form a column of larger diameter.
| Sample | 2θ (°) | d100 (nm) |
|---|---|---|
| P1 | 4.98 | 1.78 |
| P2 | 4.92 | 1.80 |
| P3 | 4.87 | 1.81 |
| P4 | 4.80 | 1.84 |
| P5 | 4.77 | 1.85 |
The second group was P6 and P7. The molecular packing model was changed with the molar content of PMT6S increasing to 80%. Previously, our group has proved that P8 (PMT6S) formed the stable ΦH phase and herein we do not discuss the experiment results again. The 1D WAXD powder patterns of P6 and P7 during the second heating step are shown in Fig. 6c and d, respectively. Take P7 as an example, two diffraction peaks in the low-angle region at 2θ = 4.72° (d = 1.87 nm) and 8.25° (d = 1.07 nm) were detected at room temperature. The d-spacings derived from these two peaks have the typical ratio of the q values of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31/2 and the two peaks can be assigned as arising from (100) and (110) diffraction planes, demonstrating a long-range ordered hexagonal lattice with cell parameters a = b = 2.15 nm and γ = 120°. Similar to P8, with the temperature increased these two diffraction peak positions shift slightly to low-angle due to thermal expansion. Moreover, the same peak positions were found for the two peaks in the wide-angle region, because these two peaks belong to amorphous packing of the alkyl chains and the π–π stacking of the Tp. From the 1D WAXD results, we found that the π–π stacking belonging to the second group was more intense compared to the first group. This different intensity suggests that as more flexible spacers are introduced, the main chain and Tp moieties in the side-chains offer a stronger decoupling effect, which allows Tp moieties act more independently.35 As can be observed in Table 3, with the molar content of PMT6S increased, the diameter of the column increased. Compared to P8, after copolymerization with the small molar of PMTS, the stiffness of the main chain is enhanced. Therefore, the stacking of the Tp is restricted and the diameter of P6 (2.11 nm) and P7 (2.15 nm) are smaller than P8 (2.21 nm).
31/2 and the two peaks can be assigned as arising from (100) and (110) diffraction planes, demonstrating a long-range ordered hexagonal lattice with cell parameters a = b = 2.15 nm and γ = 120°. Similar to P8, with the temperature increased these two diffraction peak positions shift slightly to low-angle due to thermal expansion. Moreover, the same peak positions were found for the two peaks in the wide-angle region, because these two peaks belong to amorphous packing of the alkyl chains and the π–π stacking of the Tp. From the 1D WAXD results, we found that the π–π stacking belonging to the second group was more intense compared to the first group. This different intensity suggests that as more flexible spacers are introduced, the main chain and Tp moieties in the side-chains offer a stronger decoupling effect, which allows Tp moieties act more independently.35 As can be observed in Table 3, with the molar content of PMT6S increased, the diameter of the column increased. Compared to P8, after copolymerization with the small molar of PMTS, the stiffness of the main chain is enhanced. Therefore, the stacking of the Tp is restricted and the diameter of P6 (2.11 nm) and P7 (2.15 nm) are smaller than P8 (2.21 nm).
| Sample | 2θ (°) | d-Spacing (nm) | Calculated diameter of the Φ (nm) |
|---|---|---|---|
| P6 | 4.80 | 1.84 | 2.11 |
| P7 | 4.72 | 1.87 | 2.15 |
| P8 | 4.62 | 1.92 | 2.21 |
The model of the columns from dynamic simulation results for the copolymers is shown in Fig. 7. From the whole tests, we found that the copolymers exhibit two different stabilized phase structures. As shown in Fig. 7, the copolymers whose molar content of PMT6S (MPMT6S) is below 75% were similar in property to PMTS and the polymethylacrylic acid main chain and the Tp DLCs side-chains wrap around the main chain to form a stable ΦN. The copolymers whose molar content of PMT6S is above 80% are similar in property to PMT6S and form a stable ΦH based on the self-organization of the Tp. Moreover, the Ti decreases with increase of the MPMT6S, which has been identified with the whole tests results.
Conclusions
High molecular weight binary random copolymer (poly(MTSx-co-MT6Sy)) systems were designed and prepared with AIBN as initiator at 75 °C. Thermal analysis showed that all copolymers had only a single glass transition, indicating a statistical microstructure for the main chain. The LC behaviors of copolymers were dependent on the content of PMT6S in the copolymers. The copolymers showed different phase behaviors, which were attributed to the competition between steric effects. The copolymers formed stable ΦN, similar to the property of PMTS, with the molar content of PMT6S below 75%. However, the copolymers with the molar content of PMT6S above 80% were similar to the property of PMT6S, presenting the symmetry ΦH. It was considered that the Tp discotic mesogens were based on decoupling between the main chain and the Tp side-chains. Compared with PMTS and PMT6S, the formation mechanism of two different LC phases indicated that the “decoupling effect” or “coupling effect” depended on the quantity of spacer. Moreover, the Ti of P6 and P7 was higher than PMT6S, suggesting that the copolymers can provide new functional materials with stabilized and finely-tuned ordered structures, which will be useful for both fundamental research and real applications.Acknowledgements
This study was financially supported by the National Nature Science Foundation of China (51373148) and the Innovation Platform Open Foundation of University of Hunan Province (CX2013B265).Notes and references
- S. Chen, X. Shu, H. L. Xie and H. L. Zhang, Polymer, 2013, 54, 3556–3565 CrossRef CAS.
- J. W. Y. Lam and B. Z. Tang, J. Polym. Sci., Part A: Polym. Chem., 2003, 41, 2607–2629 CrossRef CAS.
- L. C. Gao, Z. H. Shen, X. H. Fan and Q. F. Zhou, Polym. Chem., 2012, 3, 1947–1957 RSC.
- X. F. Chen, Z. H. Shen, X. H. Wan, X. H. Fan, E. Q. Chen and Y. G. Ma, et al., Chem. Soc. Rev., 2010, 39, 3072–3101 RSC.
- C. A. Yang, Q. Tan, G. Q. Zhong, H. L. Xie, H. L. Zhang and E. Q. Chen, et al., Polymer, 2010, 51, 422–429 CrossRef CAS.
- B. M. Rosen, M. Peterca, K. Morimitsu, A. E. Dulcey, P. Leowanawat and A. M. Resmerita, et al., J. Am. Chem. Soc., 2011, 133, 5135–5151 CrossRef CAS PubMed.
- V. Percec, E. Aqad, M. Peterca, J. G. Rudick, L. Lemon and J. C. Ronda, et al., J. Am. Chem. Soc., 2006, 128, 16365–16372 CrossRef CAS PubMed.
- J. F. Zheng, X. Liu, X. F. Chen, X. K. Ren, S. Yang and E. Q. Chen, Macro Lett., 2012, 1, 641–645 CrossRef CAS.
- C. Luo and J. U. Sommer, ACS Macro Lett., 2016, 5, 30–34 CrossRef CAS.
- V. Percec and D. Tomazos, in Comprehensive polymer science, first suppl, ed. S. L. Aggarwal and S. Russo, Pergamon Press, Oxford, 1992, ch. 14 Search PubMed.
- I. Julien, M. Raphae
![[l with combining umlaut]](https://www.rsc.org/images/entities/char_006c_0308.gif) , D. Laurent, C. Fred́eŕic, B. Harald, O. Yoann, C. Jeŕo
, D. Laurent, C. Fred́eŕic, B. Harald, O. Yoann, C. Jeŕo![[m with combining circumflex]](https://www.rsc.org/images/entities/char_006d_0302.gif) e, B. David, D. A. Gabriele, M. R. Otello, M. Luca and Z. Claudio, J. Am. Chem. Soc., 2014, 136, 2911–2920 CrossRef PubMed.
e, B. David, D. A. Gabriele, M. R. Otello, M. Luca and Z. Claudio, J. Am. Chem. Soc., 2014, 136, 2911–2920 CrossRef PubMed. - X. Chen, L. Chen, K. Yao and Y. Chen, ACS Appl. Mater. Interfaces, 2013, 5, 8321–8328 CAS.
- U. Scherf, A. Gutacker and N. Koenen, Acc. Chem. Res., 2008, 41, 1086–1097 CrossRef CAS PubMed.
- S. Sergeyev, W. Pisula and Y. H. Geerts, Chem. Soc. Rev., 2007, 36, 1902–1929 RSC.
- T. Emiliano, C. Rubeń, S. E. Gerardo, L. F. Ceśar, O. Josu, C. Silverio and E. Pablo, Inorg. Chem., 2014, 53, 3449–3455 CrossRef PubMed.
- Z. Danli, T. D. Ibtissam, X. Yiming, K. Farid, K. Navaphun, B. Martin, G. Daniel, H. Benoît, D. Bertrand, A. I. Dimitri, L. Emmanuelle, K. David, M. Fabrice and A. André-Jean, Macromolecules, 2014, 47, 1715–1731 CrossRef.
- J. F. Ban, S. Chen, C. Li, X. Z. Wang and H. L. Zhang, Polym. Chem., 2014, 5, 6558–6568 RSC.
- H. Finkelmann and A. H. Price, Philos. Trans. R. Soc. Lond. A., 1983, 30, 105–114 CrossRef.
- J. C. Dubois, P. L. Barny, M. Mauzac and C. Noel, Acta Polym., 1997, 48, 47–87 CrossRef CAS.
- L. Cui and Y. Zhao, Chem. Mater., 2004, 16, 2076–2082 CrossRef CAS.
- H. Tang, Z. Zhu, X. H. Wan, X. F. Chen and Q. F. Zhou, Macromolecules, 2006, 39, 6887–6897 CrossRef CAS.
- Y. H. Cheng, W. P. Chen, Z. Shen, X. H. Fan, M. F. Zhu and Q. F. Zhou, Macromolecules, 2011, 44, 1429–1437 CrossRef CAS.
- D. Tirrell, Encyclopedia of polymer science and engineering, John Wiley, New York, 1986, Vol. 4 Search PubMed.
- Y. F. Zhao, Y. Yi, X. H. Fan, X. F. Chen, X. H. Wan and Q. F. Zhou, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 2666–2674 CrossRef CAS.
- H. Tang, Z. Zhu, X. H. Wan, X. F. Chen and Q. F. Zhou, Macromolecules, 2006, 39, 6887–6897 CrossRef CAS.
- V. Percec, M. Lee and H. Jonsson, J. Polym. Sci., Part A: Polym. Chem., 1991, 29, 327–337 CrossRef CAS.
- V. Percec and M. Lee, Macromolecules, 1991, 24, 2780–2788 CrossRef CAS.
- J. F. Ban, S. Chen and H. L. Zhang, RSC Adv., 2014, 4, 54158–54167 RSC.
- J. F. Ban, S. Chen and H. L. Zhang, Chin. J. Polym. Sci., 2015, 33, 1245–1259 CrossRef CAS.
- N. Boden, R. J. Bushby and Z. B. Lu, Liq. Cryst., 1998, 25, 47–58 CrossRef CAS.
- H. L. Xie, S. J. Wang, G. Q. Zhong, Y. X. Liu, H. L. Zhang and E. Q. Chen, Macromolecules, 2011, 44, 7600–7609 CrossRef CAS.
- I. Paraschiv, K. de-Lange, M. Giesbers, L. B. van, F. C. Grozema, R. D. Abellon, L. D. A. Siebbeles, E. J. R. Sudholter, H. Zuilhof and A. T. M. Marcelis, J. Mater. Chem., 2008, 18, 5475–5481 RSC.
- M. Werth and H. W. Spiess, Makromol. Chem., Rapid Commun., 1993, 14, 329–338 CrossRef CAS.
- B. Wu, B. Mu, S. Wang, J. Duan, J. Fang, R. Cheng and D. Chen, Macromolecules, 2013, 46, 2916–2929 CrossRef CAS.
- I. Paraschiv, K. de-Lange, M. Giesbers, B. van-Lagen, F. C. Grozema, R. D. Abellon, L. D. A. Siebbeles, E. J. R. Sudholter, H. Zuilhof and A. T. M. Marcelis, J. Mater. Chem., 2008, 18, 5475–5481 RSC.
| This journal is © The Royal Society of Chemistry 2016 |