Facile one-step synthesis of highly graphitized hierarchical porous carbon nanosheets with large surface area and high capacity for lithium storage†
Abstract
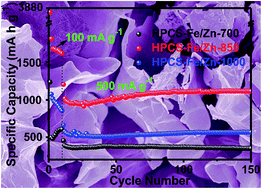
Hierarchical porous carbon nanosheets (HPCSs) were prepared from acrylic resin using FeCl3·6H2O as catalyst and ZnCl2 as activator. The as-obtained materials possess a nanosheet structure and a combination of high graphitization degree and large surface area (2109 m2 g−1). When incorporated into the anode of a Lithium Ion Battery (LIBs), the HPCSs achieve a maximum specific capacity of 1642 mA h g−1 at 100 mA g−1 and 1100 mA h g−1 at 500 mA g−1 after 150 cycles, exhibiting enormous potential in developing advanced high performance LIBs. This work offers a facile strategy for the preparation of hierarchical porous carbon materials with high performance in LIBs from resin.

 Please wait while we load your content...
Please wait while we load your content...