Dendrimeric based microbicides against sexual transmitted infections associated to heparan sulfate
Abstract
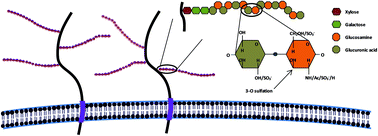
Cell surface heparan sulfate (HS) represents a common link that many sexually transmitted infections (STIs) require for infection. The role of HS is associated with several viral STIs, which include those caused by herpes simplex virus (HVS), human immunodeficiency virus (HIV), human papillomavirus (HPV) and hepatitis C virus (HCV). Nowadays, no cure has been found for any of the STIs associated with viral pathogens. In this review, we evaluate dendrimers such as peptide derivatized-dendrimers, carbosilane dendrimers, polysulfated galactose functionalized glycodendrimers and PAMAM dendrimers, among others, as potential candidates for the development of topical microbicides against viral STIs associated with HS. Subsequently, we propose the mechanism of action of these dendrimers and how it might be associated with the relevance of HS in several STIs. HS in just an ancillary factor in the case of HIV-1 infection and it plays a major role in the case of HCV, HSV-2 and HPV viruses. However, the mechanism of action presented by these dendrimers is common in all pathologies, acting at the level of viral entry into the target cell, either directly blocking the viral particles that are meant to bind to the HS or binding to cellular co-receptors.

 Please wait while we load your content...
Please wait while we load your content...