Three-dimensional potassium niobate nanoarray on vermiculite for high-performance photocatalyst fabricated by an in situ hydrothermal process†
Abstract
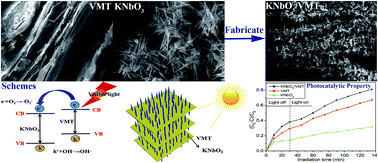
Three-dimensional (3D) potassium niobate nanoarray/vermiculite (KNbO3/VMT) was synthesized by an in situ hydrothermal method using niobium chloride as the niobium resource. Scanning electron microscopy, high resolution transmission electron microscopy, X-ray diffraction, Fourier transform infrared and X-ray photoelectron spectroscopy tests were used to confirm that KNbO3 nanoneedles have been grown both on the outer and inner surfaces of natural layered VMT and the growth mechanism of the well-aligned KNbO3 nanoarray grown on mineral VMT was attributed to the existence of Nb species. The photocatalytic performance of the as-prepared composite was investigated using the photodegradation of methylene blue (MB) under illumination. MB was removed from aqueous solution by fully taking advantage of the good absorption property of VMT and photocatalytic property of KNbO3, and visible light was used in the process. After illumination for 105 min, the removal rate of MB in aqueous solution could be higher than 81%. The removal of MB via adsorption–degradation synergy of the structured composite was much better than that of pristine VMT and KNbO3 powder with the same amount of addition, respectively. Moreover, the environmentally friendly KNbO3/VMT material is easy to synthesize and is expected to be a promising structured photocatalyst for the removal of dyes.

 Please wait while we load your content...
Please wait while we load your content...