Rhodamine-based ratiometric fluorescent probes based on excitation energy transfer mechanisms: construction and applications in ratiometric sensing
Abstract
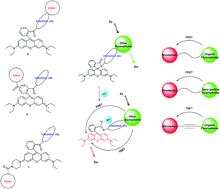
Ratiometric fluorescent probes allow the simultaneous measurement of two fluorescence signals at different wavelengths followed by calculation of their intensity ratio, which can provide more precise measurement results than intensity-based fluorescent probes. Excitation energy transfer is widely used in the design of ratiometric fluorescent probes. Rhodamine is a convenient platform for the construction of “OFF–ON” ratiometric chemosensors. Rhodamine-based ratiometric fluorescent probes based on the excitation energy transfer mechanism can be constructed by conjugated or non-conjugated connections with other chromophores. In this review, we summarized the recent advances regarding rhodamine-based ratiometric fluorescent probes based on excitation energy transfer. We reviewed these probes according to the classification of “through-space” and “through-bond” probes; we focused on the contributions of different donor fluorophores and the types of connections between the energy donors and acceptors.

 Please wait while we load your content...
Please wait while we load your content...