An important prerequisite for efficient Förster resonance energy transfer (FRET) from human serum albumin to alkyl gallate†
Qing Wang,
Ying Xiao,
Yanmei Huang and
Hui Li*
College of Chemical Engineering, Sichuan University, Chengdu, Sichuan, China. E-mail: lihuilab@sina.com; Fax: +86 028 85401207; Tel: +86 028 85405149
First published on 5th April 2016
Abstract
Förster resonance energy transfer (FRET), originally described by Förster in the 1940s, results from long-range dipole–dipole interactions between a donor and an acceptor molecule. In this work, human serum albumin (HSA) was selected as donor and the alkyl gallates were selected as acceptors. In the presence of HSA, the alkyl gallates yielded different levels of fluorescence enhancement. The corresponding energy transfer efficiency at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were 0.7% (gallic acid), 0.2% (methyl gallate), 2.4% (ethyl gallate), 20.9% (propyl gallate), and 33.1% (butyl gallate). The alkyl gallates shared the same main binding site (located in Sudlow site II) in HSA. The formation of a stable complex by fixing alkyl gallate to tryptophan of HSA at an appropriate orientation and distance was an important prerequisite for efficient FRET. Mant factors, including the factors influencing the complex formation, can hinder energy transfer. Energy transfer was seriously hindered when fatty acids preexisted in HSA. During energy transfer, some energy were dissipated as heat. Energy transfer did not happen when the donor was egg white lysozyme rather than HSA. FRET does not exhibit specificity, but the specific structure of HSA helped provide the selectivity of alkyl gallate.
1 were 0.7% (gallic acid), 0.2% (methyl gallate), 2.4% (ethyl gallate), 20.9% (propyl gallate), and 33.1% (butyl gallate). The alkyl gallates shared the same main binding site (located in Sudlow site II) in HSA. The formation of a stable complex by fixing alkyl gallate to tryptophan of HSA at an appropriate orientation and distance was an important prerequisite for efficient FRET. Mant factors, including the factors influencing the complex formation, can hinder energy transfer. Energy transfer was seriously hindered when fatty acids preexisted in HSA. During energy transfer, some energy were dissipated as heat. Energy transfer did not happen when the donor was egg white lysozyme rather than HSA. FRET does not exhibit specificity, but the specific structure of HSA helped provide the selectivity of alkyl gallate.
1. Introduction
Förster resonance energy transfer (FRET), originally described by Förster in the 1940s, results from long-range dipole–dipole interactions between a donor and an acceptor molecule.1 It is an electrodynamic phenomenon widely employed in fluorescent applications, including medical diagnostics, DNA analysis, and optical imaging.2–5 Energy transfer can be characterized by the efficiency of the transfer, which gives the probability for the donor excitation energy to be transferred to the acceptor. The transfer efficiency depends non-linearly on the distance between the donor and the acceptor. FRET thus is commonly applied to measure distances between donors and acceptors over the last 50 years or so.6 In addition, the molecular recognition and binding of ligands by highly sensitive and selective proteins are of great importance to all biomolecular processes.7–9 Using the FRET mechanism, researchers developed highly efficient molecular probes to detect proteins, such as human serum albumin (HSA).10–12 In the 1970s, Sudlow et al.13,14 used fluorescence probes found two specific and widely recognized drug-binding sites in HSA. Subsequent crystallographic studies confirmed their conclusion.15,16 Differently, the later method shows a ground-state complex was formed. FRET seems has necessary relation with the complex formation.Several methods are widely used in FRET measurements, including detecting the quenching of donor fluorescence in the presence of acceptors or measuring the fluorescence lifetime of the donor, etc.17 However, a variety of molecular interactions can decrease the fluorescence in biological systems. These interactions include excited-state reactions, molecular rearrangements, energy transfer, ground-state complex formation, and collisional quenching.6 If the ground-state complex is a nonfluorescent complex, the fluorescence quenching of HSA will becomes more complex. The original description of FRET is only valid for the interaction of one donor with one acceptor. In theory, FRET should occurs before fluorescence quenching caused by the nonfluorescent complex formation because it occurs over large distances. But both interactions occur are entirely possible as complex formation.
In this work, we focused on study the relationship between FRET and complex formation in some fluorescence probes–protein interactions. The sole tryptophan residue in HSA (Trp-214) was the single donor. Gallic acid (G), methyl gallate (MG), ethyl gallate (EG), propyl gallate (PG), and butyl gallate (BG) were used as probes (Scheme 1). HSA (contains fatty acids) and egg white lysozyme (LYZ) were chosen as comparison. The results of this study may provide valuable information in-depth understand efficient FRET in biological systems.
 | ||
| Scheme 1 Molecular structure of gallic acid (G), methyl gallate (MG), ethyl gallate (EG), propyl gallate (PG), and butyl gallate (BG). | ||
2. Materials and methods
2.1 Reagents
HSA (A1887), HSA (A1653), deuterium oxide (D2O, 99.9% D atom), and DMSO-d6 (99.9% D atom) were purchased from Sigma-Aldrich (St. Louis, USA). HSA (A1887) was essentially free from fatty acids (≤0.007% fatty acids), which was the biggest difference with HSA (A1653). The concentrations of HSA (A1887) and HSA (A1653) in phosphate buffer (pH = 7.40) were prepared using its listed molecular weight of 66.5 kDa, and the final concentrations were checked by comparing the measured absorbance with the published value: A279 = 0.531 (1 g L−1).18 LYZ was purchased from Beijing Solarbio Science and Technology Co., Ltd. (Beijing, China). The concentrations of LYZ in the phosphate buffer (pH = 7.40) were prepared using its listed molecular weight of 14.4 kDa, and the final concentration was determined using protein concentration of (1 g L−1) = 1.45A280 − 0.74A260.19 G, MG, EG, PG, BG, warfarin (WF), phenylbutazone (PB), ibuprofen (IB), dansylamide, and dansylsarcosine were purchased from J&K Scientific, Ltd. (Beijing, China).2.2 Fluorescence spectroscopy study
Fluorescence analyses were conducted on a Cary Eclipse fluorescence spectrophotometer (Varian, USA) equipped with a 1 cm quartz cell. Fluorescence spectra were obtained at 298 K and measured with 10/5 nm (excitation/emission) slit widths.2.3 Fluorescence life time measurements
Fluorescence lifetime measurements were obtained by time-correlated single photon counting with a FluoroLog-3 modular spectrofluorometer (Horiba, France). The time-resolved protein fluorescence quenching by the probes were recorded by fixing 280 nm as the excitation wavelength and 345 nm as the emission wavelength. The HSA (A1887)/HSA (A1653)/LYZ concentration was fixed at 2.0 μM, and G/MG/EG/PG/BG concentration was 2.0 μM at room temperature.2.4 Absorbance spectra study
Absorption spectra were obtained with a TU-1901 UV-vis spectrophotometer (Persee, Beijing, China) at room temperature.2.5 NMR study
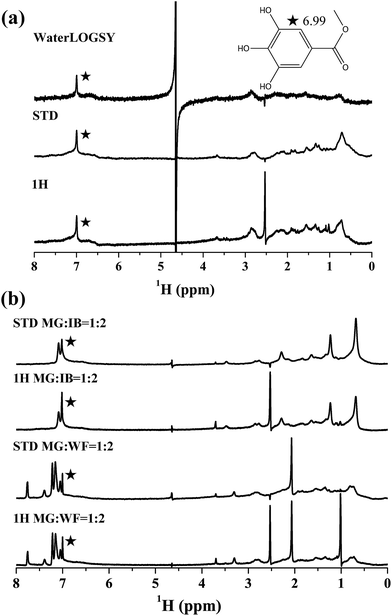
All NMR experiments were acquired on a Varian 700 MHz Inova spectrometer operating at 298 K and running under VNMRJ software (version 2.1B). The stock solutions of G/MG/EG/PG/BG (40 mM), WF (80 mM), and IB (80 mM) were prepared in DMSO-d6 for further use. The stock solution of HSA was prepared in 0.01 M PBS buffer (pH = 7.40, 50% [v/v] D2O, and H2O mixture). The final NMR samples were prepared on a sample containing 0.01 mM HSA and 0.4 mM G/MG/EG/PG/BG at room temperature. Then, competition studies were prepared on a sample containing 0.01 mM HSA, 0.4 mM G/MG/EG/PG/BG, and 0.8 mM WF/IB at room temperature. DMSO in all of the samples was less than 2% (v/v). Three 1D spectra, namely, 1H WATERGATE (w5), STD, and WaterLOGSY, were acquired for each sample. The WATERGATE spectrum was acquired for 2.4 min with the acquisition and relaxation times both set to 2 s and 32 scans. The STD experiment was acquired using the acquisition time of 1 s, 32 dummy scans, relaxation delay of 0.1 s, and a 2 s Gauss pulse train with irradiation frequency at −0.7 or −45 ppm alternatively. The total acquisition time was 15 min with 256 scans. WaterLOGSY was acquired for 15.1 min with 1 s acquisition time, 1 s relaxation, 1.3 s NOE mixing time, and 256 scans.2.6 ITC study
Calorimetric measurements were performed at 298 K by using an isothermal titration calorimeter ITC200 microcalorimeter (MicroCal; USA). Before the titration experiment was performed, all of the samples were dissolved in 0.01 M phosphate buffer (pH 7.40) and degassed properly on a Thermo Vac supplied with the calorimeter. The sample and reference cells were loaded with HSA (A1887)/HSA (A1653)/LYZ solution (20 μM) and 0.01 M phosphate buffer, respectively. Multiple injections of 2 μL of G/MG/EG/PG/BG solution (1 mM) were administered to the sample cell. The reference power and stirring speed were set at 5 μcal−1 and 750 rpm, respectively.3. Results and discussion
3.1 Significant transfer efficiency differences among the probes
At λex = 317 nm, HSA and the probes did not display obvious fluorescence in phosphate buffer. Under this condition, the enhanced fluorescence emission spectra of probes were clearly visible without the overlapping influence from the emission spectra of HSA. In the presence of HSA (2 μM), G (2 μM) still did not exhibit changes in fluorescence. The alkyl gallates (2 μM) yielded different levels of fluorescence enhancement with 1.3 ± 0.1-, 3.0 ± 0.2-, 17.1 ± 0.8-, and 25.5 ± 0.2-fold increments in the emission intensities of MG, EG, PG, and BG, respectively (Fig. 1a). Fluorescence was further enhanced as the alkyl gallate concentrations increased, especially PG and BG (Fig. 1b). The alkyl gallates were excited to different extents, and energy was transferred from HSA to alkyl gallates. FRET was slightly observed in MG but was remarkably observed in BG. FRET easily occurred as the alkyl chain length increased.We also determined the fluorescence lifetime of HSA (λex = 280 nm and λem = 345 nm) in the absence and presence of probes (Fig. 1c). The data obtained were analyzed by the tail-fitting method, and the quality of each fit was assessed by χ2 values and residuals. The mean fluorescence lifetimes (〈τ〉) for biexponential iterative fitting were calculated from the decay times and pre-exponential factors (α) by using the following equation:
| 〈τ〉 = α1τ1 + α2τ2 | (1) |
We chose the mean fluorescence lifetime as an important parameter to explore the behavior of HSA molecules bound to AP. In the absence of probes, the average lifetime of HSA (2 μM) was 5.539 ns. In the presence of probes (2 μM), the average lifetimes of HSA (Table S1†) were 5.501 ns (G), 5.526 ns (MG), 5.405 ns (EG), 4.377 ns (PG), and 3.703 ns (BG). The decay time of HSA gradually decreased as the alkyl chain length increased because more energy was consumed through energy transfer. The probes did not have a lifetime, and the lifetime of micromolecules is usually less than that of macromolecules at λex = 280 nm. Thus, the lifetime of the probes was not considered, although some probes were slightly excited (Fig. S1†). Transfer efficiencies (E) were calculated from the lifetimes of the donor in the absence (τ0) and presence (τ) of the acceptor: E = 1 − τ/τ0. The corresponding E at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were 0.7% (G), 0.2% (MG), 2.4% (EG), 20.9% (PG), and 33.1% (BG). The transfer efficiency differences among the probes were evident.
1 were 0.7% (G), 0.2% (MG), 2.4% (EG), 20.9% (PG), and 33.1% (BG). The transfer efficiency differences among the probes were evident.
3.2 Effect of various factors
According to Förster's original theory, E depends on (1) the quantum yield of a donor (QD), (2) the extent of the spectral overlap of the emission spectrum of the donor with the absorption spectrum of the acceptor, (3) the distance between donor and acceptor molecules (r), and (4) the relative orientation of donor and acceptor transition dipoles (κ2).20 Considering that the same protein was used in this study, we neglected the influence of QD. Fig. 1d illustrates the overlap of the absorption spectrum of the probes and the fluorescence emission spectrum of HSA. In G, the first absorption peak occurred at λmax = 259 nm. In alkyl gallates, the first absorption peak was detected at λmax = 272 nm. G almost had no overlap with HSA. This finding may explain why G was not excited in the presence of HSA and why E was very low. By contrast, the alkyl gallates exhibited an evident overlap with HSA. However, the extent of the spectral overlap of alkyl gallates was almost consistent and thus was not related to the differences in E. Hence, the extent of the spectral overlap was a necessary but insufficient condition. FRET was still happened even we only excited the acceptor. We considered that FRET had directly happened when the probes added to HSA which did not need the donor molecule in the excited state. The emission spectrum of donor was not limited in a certain excitation wavelength. The relationship of E and r can be expressed as follows:1
 | (2) |
| R06 = 8.79 × 1025[κ2n−4QDJ(λ)] | (3) |
3.3 Identification of probes binding site in HSA
HSA can bind to various ligands because of its multiple hydrophobic binding sites, and two primary sites (site I and site II) are known key determinants of binding specificity.14,16 Using nuclear magnetic resonance (NMR) methods, it is possible to observe and characterize the interaction between biological macromolecules and ligands.7 Saturation transfer difference (STD) NMR and waterligand optimized gradient spectroscopy (WaterLOGSY) methods are commonly used.21–23 They delivers information in the form of ligand signals in the NMR spectrum. The appearance of the probe signals in saturation transfer difference (STD) and WaterLOGSY spectra indicated that the probes interacted with HSA by forming complexes (Fig. 2a and S2–S5†).21,24,25 The main binding sites of the probes in HSA were then determined through titration experiments by using reporter ligands with known binding sites. Warfarin (WF) and ibuprofen (IB) specifically bind to sites I and II, respectively. These substances were chosen to determine the main binding site of the probes. Compared with the corresponding WATERGATE spectrum, the addition of WF to the HSA/G mixture completely suppressed the STD signal intensity of G, and the addition of IB resulted in a reduction of the STD signal intensity (Fig. S6†). A competition for the same binding site resulted in the displacements of WF/IB. G could bind to WF and IB sites. Although the proton signal of MG in the benzene ring partially overlapped with that of IB in the NMR spectra (Fig. 2b), a considerable reduction of the STD signal intensity remained visible. However, the addition of WF did not influence the STD signal of MG. Hence, MG was mainly bound to the IB site. Other alkyl gallates also yielded similar results (Fig. S7–S9†).These results were further confirmed by using the method of Sudlow et al.14 Phenylbutazone (PB) was also chosen as a site I displacement probe because FRET also exists in the WF–HSA system (λex = 317 nm; Fig. S10†). IB significantly inhibited energy transfer from HSA to BG/PG/EG, but PB did not (Fig. 3). Thus, the alkyl gallates shared the same main binding site. Alkyl gallates did not arbitrarily exist in a theoretical distance in HSA. Moreover, in theory, MG could existed in any sites where BG existed. r should not affect the differences in E in this study.
 | ||
| Fig. 3 Effect of PB/IB on the fluorescence of EG/PG/BG–HSA interaction (λex = 317 nm). C(HSA) = C(EG/PG/BG/PB/IB) = 2 μM. | ||
In fact, the probes bound to HSA were in a dynamic equilibrium; otherwise, no STD signals should have appeared. The segmental motions of HSA and the probes randomized the orientations when the probes fit into the hydrophobic cavity of site II. The increased hydrophobic alkyl chain length helped alkyl gallates fit into the hydrophobic cleft. However, the chain length must be moderated. Dey et al.26 found that strong H bonding is necessary and excessive lengths inhibit the induction of fluorescence emissions because of steric hindrance that dominantly prevents their compounds from interacting with the hydrophobic cleft. They also found that strong H bonding is necessary to retain their compounds in the hydrophobic cleft. Just interacting with the hydrophobic cleft is not enough. IB can also interacts with site II but it cannot be excited. We considered that forming a stable complex by fixing the probe to an appropriately oriented tryptophan in HSA is an important prerequisite for FRET in a probe–macromolecular interaction. FRET is a through-space interaction that occurs over long distances and is insensitive to molecular factors that affect the rate and probability of contact. Nonetheless, molecular factors that can influence the ligand–macromolecular interaction and then influence resonance should be considered. In addition, although alkyl gallates formed complexes with HSA, the complexes almost could not be nonfluorescent complexes because tryptophan was relatively far away from site II.16 It should be noted that those fluorescence probes located in site I were very likely to form nonfluorescent complexes.
3.4 Influence of preexisting fatty acids in HSA on efficient FRET
Crystallographic studies revealed fatty acids contained seven binding sites distributed heterogeneously throughout HSA.27–29 These seven sites are distributed asymmetrically across all three domains of HSA, and three of them overlap with the two primary drug-binding sites. The previously enhanced fluorescence of alkyl gallates (λex = 317 nm) significantly decreased when HSA contained fatty acids (Fig. S11†). The decay time of HSA also barely changed as different probes were added (Fig. S12†). Energy transfer was seriously hindered. However, existing fatty acids did not influence BG bound to HSA because substantial heat radiation was detected through ITC (Fig. 4a). We hypothesized that the existence of fatty acids impeded the dipole–dipole interaction on the space; as a consequence, energy transfer decreased. Nonetheless, heat radiation was more evident in the absence of fatty acids in BG–HSA interaction (Fig. 4b). We considered that energy could not be totally transferred to the donor and some amounts of energy were dissipated as heat during resonance energy transfer. Fatty acids slightly affected heat radiation in low energy transfer efficiency systems, such as G/MG–HSA system (Fig. S13 and S14†). ITC relies on the detection of heat effect upon binding. Under this condition, the binding constant measured through ITC should be different from the actual value.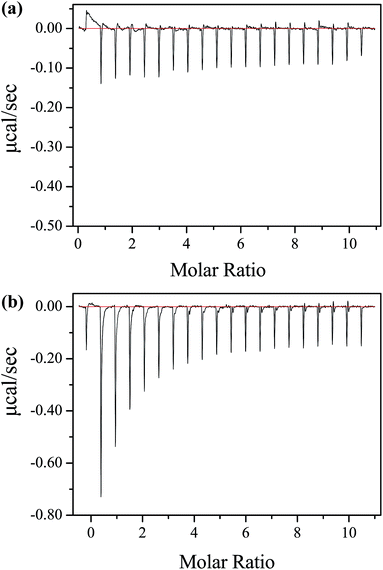 | ||
| Fig. 4 Raw data for the ITC titration of 1 mM BG with 0.02 mM (a) HSA (contain fatty acids) and (b) HSA (essentially fatty acid free) at pH 7.40 phosphate buffer and 298 K. | ||
3.5 Efficient FRET does not arbitrarily occur
LYZ was chosen and subjected to a comparative study (Fig. S15–S18†). LYZ is pervasive in various body fluids, such as tears, saliva, mucus, urine, lymphatic tissues, human milk, and cells of the innate immune system. LYZ is a single, non-glycosylated polypeptide chain that is composed of 129 amino acid residues folded in two domains.30 LYZ structure is relatively simpler than HSA but it contains six tryptophan residues that could act as potential donors.31 The fluorescence of the probes (λex = 317 nm) was not enhanced in the presence of LYZ, and the decay time of LYZ changed slightly as different probes were added. Thus no FRET occurred from LYZ to the probes. At λex = 280 nm, the probes slightly quenched LYZ; as such, no evident heat changes were detected through isothermal titration calorimetry (ITC). The probes could slightly quench the donor but the alkyl chain length of the alkyl gallates was not related to quenching. The differences among alkyl gallate–HSA interactions were solely attributed to FRET. Similar phenomena were also found in other widely recognized fluorescence probes. For example, the fluorescence in LYZ remains unchanged, although dansylamide (located in site I) and dansylsarcosine (locate in site II) show an evident fluorescence increase in HSA (Fig. S19†). FRET could not arbitrarily occur in all ligand–macromolecular interactions. However, FRET also had no specificity. For example in HSA, FRET can not only occur in sites I and II of HSA but also on the HSA surface.13,14,32 We hypothesized that the specific structure of HSA helped the selective probes fixed distance to the donor and provided a good resonance environment as FRET was a strongly distance-dependent process. Because bovine serum albumin (BSA) and HSA display approximately 76% sequence homology,33 the selective probe L15 also had highly selective in BSA.264. Conclusions
In this work, the relationship between FRET and complex formation in biological systems was clarified in alkyl gallate–HSA interactions. The transfer efficiency differences among different alkyl gallates were evident although they had only minor difference in structure. After excluding various factors, we considered that the formation of a stable complex by fixing alkyl gallate to tryptophan of HSA at an appropriate orientation and distance was an important prerequisite. If the complex is a nonfluorescent complex, interaction with HSA will becomes more complex. Many factors, including the factors influencing the complex formation, can hinder energy transfer. During energy transfer, some energy were dissipated as heat. As a result, the binding constant calculated through ITC should be different from the actual value. FRET does not exhibit specificity, but the specific structure of HSA helped provide the selectivity of alkyl gallate.Acknowledgements
This work was supported by the Applied Basic Research Project of Sichuan Province (Grant No. 2014JY0042), the Testing Platform Construction of Technology Achievement Transform of Sichuan Province (Grant No. 13CGPT0049), and the National Development and Reform Commission and Education of China (Grant No. 2014BW011). Thanks to School of Life Science, University of Science and Technology of China for NMR technical assistance.References
- T. Förster, Ann. Phys., 1948, 437, 55–75 CrossRef.
- I. L. Medintz and H. Mattoussi, Phys. Chem. Chem. Phys., 2009, 11, 17–45 RSC.
- J. Shi, F. Tian, J. Lyu and M. Yang, J. Mater. Chem. B, 2015, 3, 6989–7005 RSC.
- A. Poudel, X. Chen and M. A. Ratner, J. Phys. Chem. Lett., 2016, 7, 955–960 CrossRef CAS PubMed.
- M. Xue, W. Wei, Y. Su, D. Johnson and J. R. Heath, J. Am. Chem. Soc., 2016, 138, 3085–3093 CrossRef CAS PubMed.
- B. Prevo and E. J. Peterman, Chem. Soc. Rev., 2014, 43, 1144–1155 RSC.
- G. U. Nienhaus, Protein-Ligand Interactions, Springer, USA, 2005 Search PubMed.
- A. C. A. Roque, Ligand-Macromolecular Interactions in Drug Discovery, Springer, London, 2010 Search PubMed.
- X. Zhao, D. Lu, F. Hao and R. Liu, J. Hazard. Mater., 2015, 292, 98–107 CrossRef CAS PubMed.
- Y.-Y. Wu, W.-T. Yu, T.-C. Hou, T.-K. Liu, C.-L. Huang, I.-C. Chen and K.-T. Tan, Chem. Commun., 2014, 50, 11507–11510 RSC.
- Y. Yu, S. Y. New, J. Xie, X. Su and Y. N. Tan, Chem. Commun., 2014, 50, 13805–13808 RSC.
- X.-P. He, Y. Zang, T. D. James, J. Li and G.-R. Chen, Chem. Soc. Rev., 2015, 44, 4239–4248 RSC.
- G. Sudlow, D. Birkett and D. Wade, Mol. Pharmacol., 1975, 11, 824–832 CAS.
- G. Sudlow, D. Birkett and D. Wade, Mol. Pharmacol., 1976, 12, 1052–1061 CAS.
- X. M. He and D. C. Carter, Nature, 1992, 358, 209–215 CrossRef CAS PubMed.
- J. Ghuman, P. A. Zunszain, I. Petitpas, A. A. Bhattacharya, M. Otagiri and S. Curry, J. Mol. Biol., 2005, 353, 38–52 CrossRef CAS PubMed.
- Á. I. Fábián, T. Rente, J. Szöllősi, L. Mátyus and A. Jenei, ChemPhysChem, 2010, 11, 3713–3721 CrossRef PubMed.
- O. K. Abou-Zied and O. I. K. Al-Shihi, J. Am. Chem. Soc., 2008, 130, 10793–10801 CrossRef CAS PubMed.
- F. Yoshizaki, T. Oshima and K. Imahori, J. Biochem., 1971, 69, 1083–1089 CAS.
- J. R. Lakowicz, Principles of fluorescence spectroscopy, Springer, Singapore, 3rd edn, 2009 Search PubMed.
- M. Mayer and B. Meyer, J. Am. Chem. Soc., 2001, 123, 6108–6117 CrossRef CAS PubMed.
- C. Dalvit, G. Fogliatto, A. Stewart, M. Veronesi and B. Stockman, J. Biomol. NMR, 2001, 21, 349–359 CrossRef CAS PubMed.
- A. D. Gossert, C. Henry, M. J. Blommers, W. Jahnke and C. Fernandez, J. Biomol. NMR, 2009, 43, 211–217 CrossRef CAS PubMed.
- M. Liu, X.-a. Mao, C. Ye, H. Huang, J. K. Nicholson and J. C. Lindon, J. Magn. Reson., 1998, 132, 125–129 CrossRef CAS.
- J. Gao, R. Ma, W. Wang, N. Wang, R. Sasaki, D. Snyderman, J. Wu and K. Ruan, PLoS One, 2014, 9, e88098 Search PubMed.
- G. Dey, P. Gaur, R. Giri and S. Ghosh, Chem. Commun., 2016, 52, 1887–1890 RSC.
- S. Curry, P. Brick and N. P. Franks, BBA, Biochim. Biophys. Acta, Mol. Cell Biol. Lipids, 1999, 1441, 131–140 CrossRef CAS.
- A. A. Bhattacharya, T. Grüne and S. Curry, J. Mol. Biol., 2000, 303, 721–732 CrossRef CAS PubMed.
- J. R. Simard, P. A. Zunszain, J. A. Hamilton and S. Curry, J. Mol. Biol., 2006, 361, 336–351 CrossRef CAS PubMed.
- A. Das, R. Thakur, A. Dagar and A. Chakraborty, Phys. Chem. Chem. Phys., 2014, 16, 5368–5381 RSC.
- A. Pellegrini, U. Thomas, N. Bramaz, S. Klauser, P. Hunziker and R. Von Fellenberg, J. Appl. Microbiol., 1997, 82, 372–378 CAS.
- T. Sen, S. Mandal, S. Haldar, K. Chattopadhyay and A. Patra, J. Phys. Chem. C, 2011, 115, 24037–24044 CAS.
- K. A. Majorek, P. J. Porebski, A. Dayal, M. D. Zimmerman, K. Jablonska, A. J. Stewart, M. Chruszcz and W. Minor, Mol. Immunol., 2012, 52, 174–182 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra06920g |
| This journal is © The Royal Society of Chemistry 2016 |