Controlled synthesis of 3D hierarchical NiSe microspheres for high-performance supercapacitor design†
Kailu Guoa,
Feifei Yangb,
Shizhong Cuia,
Weihua Chen*b and
Liwei Mi*a
aCenter for Advanced Materials Research, Zhongyuan University of Technology, Zhengzhou, 450007, P. R. China. E-mail: mlwzzu@163.com
bCollege of Chemistry and Molecular Engineering, Zhengzhou University, Zhengzhou, 450001, P. R. China. E-mail: chenweih@zzu.edu.cn
First published on 5th May 2016
Abstract
In this work, hierarchical nanosheet-based NiSe microspheres were successfully fabricated using a facile one-step solvothermal method, in which ethylenediamine and N,N-dimethylformamide were used as the mixed solvent. The evolution of this morphology and the effects of cetyltrimethylammonium bromide were also explored. The as-synthesized NiSe microspheres exhibited the ideal performance when employed as electrode materials of supercapacitors.
Introduction
The energy crisis is becoming increasingly serious, which has induced an urgent demand for better energy storage devices.1,2 Therefore, supercapacitors with high power density, faster charge–discharge cycling processes, and long cycle life have been attracting considerable attention from a number of application sectors.3–5 At present, transition metal oxides, sulfides, hydroxides, conductive polymers, and carbon materials with high specific surface area are strongly investigated as the supercapacitor electrode materials with excellent properties in a three-electrode system.6–10 Moreover, the supercapacitor devices with high energy density are the bridge to the practical application of these superior materials.10,11Currently, many researchers have focused on NiO, Ni(OH)2, and NiSx for high-performance supercapacitor electrode materials, because of their low band gap and high conductivity.7,9,12 Compared with O and S, Se as the closest neighbor in the sixth main group of the periodic table exhibits better metallic property, thereby indicating more excellent electronic properties.13,14 Therefore, NiSe is also an especially promising electrode materials based on the variety of oxidation states and good electrical conductivity for charge transfer.14,15 In addition, morphology and structure control can improve the performance of electrode materials for supercapacitors.16,17 The method that synthesizes electrode materials in the three-dimensional (3D) foam metal frame can reduce the electrode preparation step and can increase specific energy and capacity.18 The morphology adjustment of these electrode materials prepared by in situ synthesis onto current collectors is mostly conducted by changing the reaction temperature, reaction time, or solvent system.19 Cetyltrimethylammonium bromide (CTAB) is a cationic surfactant, which has been widely used as stabilizer or soft template to help prepare a variety of nanoparticles.20–22 However, the mechanism of CTAB regulation applied to the synthesis of electrode materials growing onto foam metal has been rarely reported. The hierarchical 3D nanostructures of electrode materials can provide more active sites and a larger specific surface area, which are significant to enhance the electrochemical performance.18 However, to the best of our knowledge, the use of hierarchical nanosheet-based NiSe microspheres grown on nickel foam as an electrode for supercapacitors, especially for the supercapacitor devices, has not been reported previously.
In this work, we used the simple hydrothermal method to synthesize nanosheet-based NiSe microspheres on the nickel foam substrate in one step and explored the growth process of this morphology. A series of different morphologies of nickel selenide compounds were prepared and adjusted by the surfactant (CTAB) successfully. The probable reaction mechanism of CTAB was discussed in detail in this paper. These nickel selenide compounds were applied as pseudocapacitance electrode materials and were fabricated on asymmetric supercapacitor devices successfully.
Experimental section
Materials synthesis
N,N-Dimethylformamide (DMF), anhydrous ethylenediamine (EDA), selenium powder (Se), and CTAB were all purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China.All chemicals were of analytical grade and were used without further purification. In a typical preparation procedure, 14 mL DMF and 1 mL EDA, 0.029345 g Ni foam (0.8 cm × 0.8 cm; thickness: 2 mm), 0.0410 g selenium powder, and 0.1093 g (0.3 mmol) CTAB were added into a 20 mL Teflon-lined autoclave, respectively. The mixture was maintained at 160 °C for 24 h and was cooled to room temperature naturally. The product was removed from the solution and repeatedly washed with deionized water, followed by washing with C2H5OH. Finally, the as-prepared nanosheet-based NiSe microspheres were dried in a vacuum oven at 60 °C for 8 h. To explore the growth mechanism of nanosheet-based NiSe microspheres, the reaction times used to produce NiSe-2 and NiSe-3 were 16 and 20 h, respectively. The effects of CTAB on the composition and morphology of the products were also studied at the same synthesis conditions of NiSe microspheres, except that one factor was altered. The dosage of CTAB was 0.0729 g (0.2 mmol) for NiSe-5, and NiSe-4 was prepared without introducing CTAB. The post-treatment of these materials were similar to that of NiSe microspheres.
Preparation of NiSe electrode
80% of the active material and 10% each of Super P and polytetrafluoroethylene (PTFE) were uniformly mixed in an appropriate amount of isopropyl alcohol and then pressed into a thin sheet, which was used as a working electrode. The mass of the active material in each sheet was approximately 15 mg.Preparation of activated carbon electrode
The negative electrode was fabricated by the scraper method. Activated carbon (AC) (50 wt%) and polyvinylidene fluoride (PVDF) (50 wt%) were mixed with a little ethanol to form a homogeneous paste. This paste was successively daubed on NF with a size similar to that of the as-obtained samples until the gap of the foam was completely filled. After being dried at 80 °C for 12 h, an AC electrode was obtained. The quality of the activated carbon is about 33 mg cm−2.Characterization
The morphology of the end products were investigated by scanning electron microscopy (SEM) equipped with an energy-dispersive X-ray (EDX) spectroscopy system. The phase purity of the end products were identified by X-ray diffraction (XRD) with a Bruker D8 Advance X-ray powder diffractometer using Cu-Kα irradiation at a scan rate of 0.1° s−1. All XRD measurements were performed within 20° ≤ 2θ ≤ 80°. The nanostructures of the resulting samples were recorded with a JEOL JEM-2010 transmission electron microscope (TEM). The X-ray photoelectron spectroscopy (XPS) of the as-obtained NiSe microspheres was performed using a Kratos AXIS ULTRA X-ray photoelectron microscope with Al-Kα X-rays as the excitation source.Electrochemical performance testing
The electrochemical properties of the nanosheet-based NiSe microspheres were first evaluated by using standard three-electrode systems (using NiSe microspheres as working electrode, Hg/HgO as reference electrode, and nickel strip as a counter electrode). Cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS) and galvanostatic charge–discharge measurement were conducted in 2 M KOH aqueous solution.The electrochemical properties of the nanosheet-based NiSe microspheres electrode were further investigated by fabricating asymmetric supercapacitor devices. The asymmetric supercapacitor was assembled using the prepared nanosheet-based NiSe microspheres as the positive electrode, activated carbon as the negative electrode, non-woven fabric material as the separator, and 2 M KOH solution as the electrolyte. The CV analysis and galvanostatic charge–discharge measurement of the asymmetric supercapacitor were performed on an electrochemical workstation.
Results and discussion
The hierarchical nanosheet-based NiSe microspheres were synthesized on a piece of nickel foam substrate by one pot in situ growth method. As shown in Fig. 1a, the low-magnification SEM image of the as-obtained NiSe microspheres revealed that the nickel foam with a 3D framework was kept intact. In addition, the surface of nickel template was densely covered with a layer of the end products. The magnified SEM image of the NiSe microspheres was shown in Fig. 1b. The SEM image showed that the diameter of the individual microsphere was nearly 1 μm, and the surface of these microspheres were composed of nano-thin-sheets (approximately 20 nm thickness). Between these nanosheets, numerous micropores with nearly 200 nm width were found. This porous structure could increase the contact area of the electrolyte, which was helpful in improving its electrochemical properties. Furthermore, TEM was employed to characterize the detailed morphology and structural characteristics of the NiSe microspheres. A layer of very thin nanosheets had covered the surface of NiSe microspheres (Fig. 1c). Moreover, the high-resolution TEM (HRTEM) images were used to illustrate the geometrical structure of these nanosheets on the NiSe microspheres surface. The selected area electron diffraction pattern confirmed the single crystallinity of the NiSe nanosheets (Fig. 1d). The 0.272 nm and 0.317 nm lattice spacing corresponded to the 101 and 100 planes of the NiSe phase. The HRTEM image of the nanosheet displayed clear crystal lattice, which revealed the high crystallinity. The distinct fringe spacing was 2.72 Å indexed to the 101 plane of NiSe (Fig. 1d inset). | ||
| Fig. 1 Characterization of the NiSe microspheres: (a) low-magnification SEM image, (b) high-magnification SEM image, (c) TEM image of a NiSe microspheres and (d) SAED pattern (inset: HRTEM image). | ||
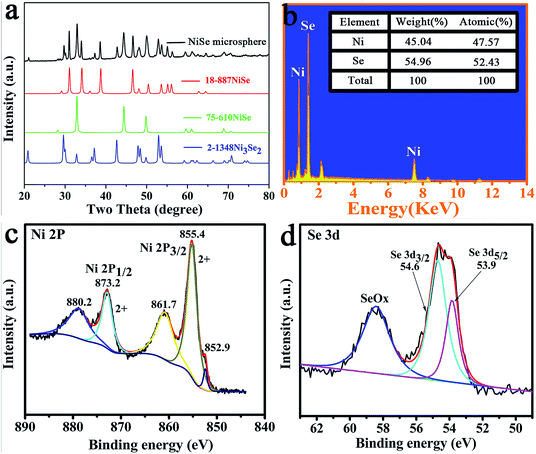
The phase composition and crystal structure of products were examined using XRD analysis. The XRD pattern of NiSe microspheres was shown in Fig. 2a. The XRD pattern showed that all the diffraction peaks were consistent with the standard cards (NiSe JCPDS card no. 18-887, NiSe JCPDS 75-610, and Ni3Se2 JCPDS card no. 2-1348). Therefore, NiSe microspheres might be a mixture of NiSe and a spot of Ni3Se2. Few Ni peaks were found in the figure, which indicated that the nickel substrate was nearly converted into the products. The strong and sharp diffraction peaks implied that the obtained products were significantly crystallized. EDX analysis was employed for the NiSe microspheres (Fig. 2b). The elemental ratio of Ni and Se in the microspheres was 52.43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47.57, which was a close ratio to 1
47.57, which was a close ratio to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The XPS spectra are shown in Fig. 2c and d. Fig. 2c shows that the Ni2p3/2 and Ni2p1/2 peaks appeared at 855.4 and 873.2 eV, respectively. These peaks had two shakeup satellites at 861.7 and 880.2 eV, which indicated that Ni was in the Ni2+ oxidation state.14,23 The peak at 852.9 eV was assigned to metallic Ni2p, which came from unreacted nickel substrate.14,24 This result was in good agreement with the XRD pattern. The Se3d features were shown in Fig. 2d. The peaks at 54.6 and 53.9 eV represented the binding energy of Se3d3/2 and 3d5/2, respectively. The peak at 58.7 eV could be assigned to the oxidized Se.14,25
1. The XPS spectra are shown in Fig. 2c and d. Fig. 2c shows that the Ni2p3/2 and Ni2p1/2 peaks appeared at 855.4 and 873.2 eV, respectively. These peaks had two shakeup satellites at 861.7 and 880.2 eV, which indicated that Ni was in the Ni2+ oxidation state.14,23 The peak at 852.9 eV was assigned to metallic Ni2p, which came from unreacted nickel substrate.14,24 This result was in good agreement with the XRD pattern. The Se3d features were shown in Fig. 2d. The peaks at 54.6 and 53.9 eV represented the binding energy of Se3d3/2 and 3d5/2, respectively. The peak at 58.7 eV could be assigned to the oxidized Se.14,25
 | ||
| Fig. 2 Characterization of the NiSe microspheres: (a) XRD pattern; (b) EDX analysis, XPS spectra of (c) Ni2p and (d) Se3d regions. | ||
To explore the growth mechanism of the hierarchical nanosheet-based NiSe microspheres, we adjusted the reaction parameters. In the same solvent system and reaction temperature, reaction time had a significant influence on the morphology of nickel selenide materials. Therefore, NiSe-2 and NiSe-3 were synthesized by using the same conditions as NiSe microspheres, except for the reaction time. The reaction time of NiSe-2 and NiSe-3 was 16 h and 20 h, respectively.
With increasing reaction time, the NiSe on the surface of nickel foam was transformed from the initial sheet structure to the nanosheet-based microspheres. Fig. 3a shows the SEM image of NiSe-2, which consisted of many irregular, petal-like NiSe nanosheets that were less than 100 nm. When the reaction time was increased to 20 h (NiSe-3, Fig. 3b), these nanosheets were gradually assembled into NiSe microspheres with diameters of approximately 1 μm. When the reaction time was further extended to 24 h, the nanosheet-based microspheres were successfully prepared with uniform size and distribution (NiSe microspheres, Fig. 3c). As shown in Fig. 3f, the schematic of morphology evolution explained this process in detail. The XRD patterns of these samples are shown in Fig. 3d. The results exhibited that the compositions of these products were similar to those of NiSe microspheres corresponding to the standard data for NiSe JCPDS card no. 18-887, NiSe JCPDS 75-610, and Ni3Se2 JCPDS card no. 2-1348. Furthermore, with the increase of reaction time, the peaks of the nickel base gradually weakened and the peaks of Ni3Se2 gradually strengthened. According to the EDX results, with the extension of reaction time, the amount of Se on the nickel foam skeleton increased gradually. When the reaction was 24 h, the molar ratio of nickel to selenium on the surface of nickel foam was close to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 3e).
1 (Fig. 3e).
As a cationic surfactant, CTAB played an important role in controlling the formation of micro- and nano-architectures. Accordingly, we constantly adjusted the dosage of CTAB to better understand the acting mechanism in the process of forming NiSe microspheres. The SEM images of the NiSe with or without CTAB were shown in Fig. 4. Fig. 4a represents the SEM micrographs of NiSe-4 prepared without CTAB. Notably, nickel foam skeleton was significantly preserved, but a few wrinkles and protuberances were found on the surface of nickel substrate without any characteristic morphology. As shown in Fig. 4b, a layer of stone-like products had uniformly covered the surface of nickel foam when 0.0729 g CTAB was added to NiSe-5. Then, when the dosage of CTAB was increased to 0.1093 g, NiSe nano-stones had converted into microspheres (Fig. 4c) that covered a series of nanosheets on the surface. The XRD patterns of these products are shown in Fig. 4d. After the analysis, the diffraction peaks were perfectly indexed to NiSe JCPDS card no. 18-887, NiSe JCPDS 75-610, and Ni3Se2 JCPDS card no. 2-1348. However, when the added amount of CTAB was changed, the intensity of the diffraction peaks of some crystallographic faces had also changed. This phenomenon might be attributed to the promotion or inhibition of the surfactants on certain crystallographic facets.22
According to the previously described action mechanism of the surfactants, the growth of nanostructures was related to the selective absorption of organic surfactants on certain crystallographic facets.22,26 This selective adsorption could control the growth rate of various crystallographic faces or could induce them for ordered self-assembly. The surfactants could also be adsorbed on the surface of the particles to prevent the nanoparticles from being aggregated into larger particles.27,28 On the basis of the proposed mechanism of the CTAB, we proposed a new possible reaction mechanism for the synthesis of the hierarchical nanosheet-based NiSe microspheres (Fig. 4e). This mechanism was related to the process of Se ion etching of nickel foam under the control of CTAB. As a cationic surfactant, CTAB would be ionized for CTA+ and Br− in our given solvent. In the process of reaction, CTA+ would combine with the selenium ions in the solvent system, which, in turn, would form the CTA+ Se−. Some of reactive sites on the surface of nickel foam had a priority to react with selenium ions, which would erode nickel foam from the exterior to the interior gradually. Hence, stone-like irregular block was formed. As the dosage of CTAB was increased, parts of CTA+ Se− would continue to combine with active sites on the block surface. As the reaction was conducted, these blocks would be further eroded. Finally, the surface of these blocks had been corroded into numerous nanosheets.
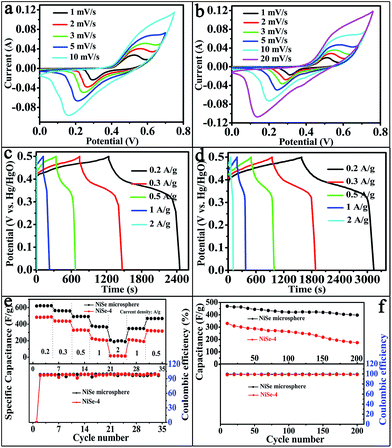
To investigate the electrochemical performances of the as-prepared materials, we performed CV tests using 2 M KOH as an electrolyte with different scan rates. Fig. 5a and b showed the CV curves of NiSe-4 electrode and NiSe microspheres electrode respectively. A pair of peaks was visible in each voltammogram, indicating that the measured capacitance was mainly from redox mechanism. When the scan rate was increased, the shape of the curves was maintained, and the peak current increased, suggesting that all electrodes had excellent rate performance and could result in rapid redox reactions. Whereas, the redox peaks almost disappeared at a scan rate of 10 mV s−1 for NiSe-4, and 20 mV s−1 for NiSe microspheres, which indicated that NiSe microspheres had better rate performance compared with NiSe-4. The redox mechanism of NiSe in aqueous alkaline solution might be explained in the following mechanism:7,14
| NiSe + H2O + 1/2O2 → Ni(OH)2 + Se, | (1) |
| Ni(OH)2 + OH− ↔ NiOOH + H2O + e−. | (2) |
To study the specific capacitance and evaluate the rate capability of the NiSe-4 and the nanosheet-based NiSe microspheres, galvanostatic charge–discharge measurements were performed in 2 M KOH solution at various current densities, as shown in Fig. 5c and d. The capacitance value was derived from the discharge curves according to eqn (3):
| C = IΔt/mΔV, | (3) |
In order to evaluate the cyclic stability of NiSe microspheres electrode and NiSe-4 electrode, a continuous charging and discharging process was carried out. As shown in Fig. 5f, under a constant current density of 0.5 A g−1, the electrode was performed for 200 times charge–discharge tests in 2 M KOH solution with three-electrode construction. The NiSe microspheres electrode and NiSe-4 electrode capacitance value remained 84.6% and 53% of initial specific capacitance after 200 times cycles respectively, indicating NiSe microspheres were potential candidates for supercapacitors with better electrochemical properties.
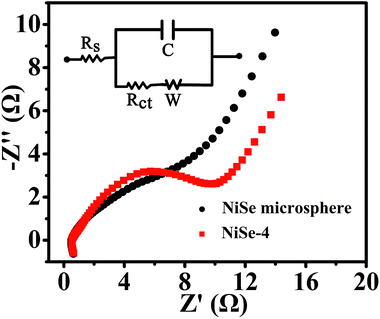
Electrochemical impedance spectroscopy (EIS) was a compelling evidence to describe the electrochemical properties related to ion diffusion and charge transfer process of supercapacitor electrode. Fig. 6 showed the Nyquist plots of NiSe microspheres electrode and NiSe-4 electrode obtained from the open circuit potential in the frequency range from 0.01 to 105 Hz which consisted of a semicircle and a straight line at the high- and low-frequency region, respectively. It could be observed obviously that NiSe microspheres exhibited the same electrochemical system resistance (Rs) and the smaller charge-transfer resistance (Rct). Thus, the hierarchically typical nanosheet structure made NiSe microspheres achieve the better electrochemical performance.
To evaluate the nanosheet-based NiSe microspheres for practical application, an asymmetric supercapacitor was fabricated by using as-prepared NiSe and activated carbon as the positive and negative electrode, respectively. The electrochemical performance of AC was evaluated by CV (Fig. S1, ESI†). The CV curve of the AC which was similar to rectangular indicated that the capacity of activated carbon was attributed to the electric double layer. In addition, the potential window of AC electrode was −1 to 0 V (vs. Hg/HgO), indicating the potential window could be extended to 1.5 V by assembling an asymmetric supercapacitor with AC as the negative electrode and NiSe as the positive electrode.
The CV curves of the two-electrode supercapacitor devices of NiSe-4 and NiSe microspheres at different scan rates were depicted in Fig. 7a and b. The shape of these CV curves clearly revealed the pseudocapacitive characteristics, and a pair of redox peaks could be observed. The redox reaction peaks were attributed to the redox reaction based on Ni2+/Ni3+. Galvanostatic charge–discharge measurement was performed at various current densities, as shown in Fig. 7c and d, to estimate the performance of the asymmetric supercapacitor. The specific capacitance of the supercapacitor cell was calculated based on eqn (3), but m was changed to the total mass of the active materials of the two electrodes. The calculated specific capacitances (Fig. 7e) of the supercapacitor cells based on NiSe microspheres and NiSe-4 were 80 and 61 F g−1 at a current density of 0.2 A g−1. Therefore, the nanosheet-based NiSe microspheres exhibited superior electrochemical activity compared with NiSe-4.
The cycle stability was also an important requirement for the asymmetric supercapacitor. As shown in Fig. 7f, the cycling life of the asymmetric supercapacitor was performed by repeating the charge–discharge cycling between 0 and 1.5 V at a constant current density of 0.5 A g−1 over 2000 cycles. After 2000 cycles, the specific capacitances of the two kinds of asymmetric supercapacitors could maintain 40 and 32 F g−1 at a current density of 0.5 A g−1, respectively. Moreover, the coulombic efficiency of these asymmetric supercapacitors remained at nearly 100% during the charge–discharge cycling process. These results proved that the nanosheet-based NiSe microspheres which were used as the electrode materials for asymmetric supercapacitor devices were promising.
Conclusions
In this paper, we reported a facile one-step method to prepare the hierarchical nanosheet-based NiSe microspheres. The evolution of this morphology was also successfully explored through regulating the reaction time. By adjusting the dosage of CTAB, the morphology of NiSe could be controlled. We proposed a new possible reaction mechanism to explain the role of CTAB. The nanosheet-based NiSe microspheres were used as a supercapacitor electrode material, which exhibited ideal electrochemical performance (492 F g−1 at a current density of 0.5 A g−1). In addition, an asymmetric supercapacitor was successfully fabricated used the as-prepared materials, which showed the desired electrochemical properties (80 F g−1 at a current density of 0.2 A g−1). Furthermore, this work enriched the type of electrode materials and provided guidance for the practical application of NiSe electrode materials.Acknowledgements
This work was supported by the Natural Science Foundation of China (No. 21443003 & U1407103), Henan Province (No. 15HASTIT003), Innovation Scientists and Technicians Troop Construction Projects of Henan Province and Zhengzhou University (No. 1421316035). We appreciate Dr Wutao Wei, Prof. Chuntai Liu and Prof. Changyu Shen for their help to modify this article.Notes and references
- D. P. Dubal, O. Ayyad, V. Ruiz and P. Gómez-Romero, Hybrid energy storage: the merging of battery and supercapacitor chemistries, Chem. Soc. Rev., 2015, 44, 1777–1790 RSC.
- Y. R. Zhu, Z. B. Wu, M. J. Jing, H. S. Hou, Y. C. Yang, Y. Zhang, X. M. Yang, W. X. Song, X. N. Jia and X. B. Ji, Porous NiCo2O4 spheres tuned through carbon quantum dots utilised as advanced materials for an asymmetric supercapacitor, J. Mater. Chem. A, 2015, 3, 866–877 CAS.
- C. Liu, F. Li, L. P. Ma and H. M. Cheng, Advanced Materials for Energy Storage, Adv. Mater., 2010, 22, E28–E62 CrossRef CAS PubMed.
- A. S. Westover, J. W. Tian, S. Bernath, L. Oakes, R. Edwards, F. N. Shabab, S. Chatterjee, A. V. Anilkumar and C. L. Pint, A Multifunctional Load-Bearing Solid-State Supercapacitor, Nano Lett., 2014, 14, 3197–3202 CrossRef CAS PubMed.
- G. H. Yu, L. B. Hu, N. Liu, H. L. Wang, M. Vosgueritchian, Y. Yang, Y. Cui and Z. N. Bao, Enhancing the Supercapacitor Performance of Graphene/MnO2 Nanostructured Electrodes by Conductive Wrapping, Nano Lett., 2011, 11, 4438–4442 CrossRef CAS PubMed.
- J. Kang, A. Hirata, L. Kang, X. Zhang, Y. Hou, L. Chen, C. Li, T. Fujita, K. Akagi and M. Chen, Enhanced Supercapacitor Performance of MnO2 by Atomic Doping, Angew. Chem., Int. Ed., 2013, 125, 1708–1711 CrossRef.
- W. T. Wei, L. W. Mi, Y. Gao, Z. Zheng, W. H. Chen and X. X. Guan, Partial Ion-Exchange of Nickel-Sulfide-Derived Electrodes for High Performance Supercapacitors, Chem. Mater., 2014, 26, 3418–3426 CrossRef CAS.
- Y. Xu, X. Huang, Z. Lin, X. Zhong, Y. Huang and X. Duan, One-Step Strategy to Graphene/Ni(OH)2 Composite Hydrogels as Advanced Three-Dimensional Supercapacitor Electrode Materials, J. Nano Res., 2013, 6, 65–76 CrossRef CAS.
- H. L. Wang, H. S. Casalongue, Y. Y. Liang and H. J. Dai, Ni(OH)2 Nanoplates Grown on Graphene as Advanced Electrochemical Pseudocapacitor Materials, J. Am. Chem. Soc., 2010, 132, 7472–7477 CrossRef CAS PubMed.
- L. W. Mi, W. T. Wei, S. B. Huang, S. Z. Cui, W. X. Zhang, H. W. Hou and W. H. Chen, Nest-like Ni@Ni1.4Co1.6S2 Electrode for Flexible High-Performance Rolling Supercapacitors Device Design, J. Mater. Chem. A, 2015, 3, 20973–20982 CAS.
- X. Wang, A. Sumboja, M. F. Lin, J. Yan and P. S. Lee, Enhancing electrochemical reaction sites in nickel–cobalt layered double hydroxides on zinc tin oxide nanowires: a hybrid material for an asymmetric supercapacitor device, Nanoscale, 2012, 4, 7266–7272 RSC.
- C. H. Wu, S. X. Deng, H. Wang, Y. X. Sun, J. B. Liu and H. Yan, Preparation of Novel Three-Dimensional NiO/Ultrathin Derived Graphene Hybrid for Supercapacitor Applications, ACS Appl. Mater. Interfaces, 2014, 6, 1106–1112 CAS.
- L. W. Mi, H. Sun, Q. Ding, W. H. Chen, C. T. Liu, H. W. Hou, Z. Zheng and C. Y. Shen, 3D hierarchically patterned tubular NiSe with nano-/microstructures for Li ion battery design, Dalton Trans., 2012, 41, 12595–12600 RSC.
- C. Tang, Z. H. Pu, Q. Liu, A. M. Asiri, X. P. Sun, Y. L. Luo and Y. Q. He, In Situ Growth of NiSe Nanowire Film on Nickel Foam as an Electrode for High-Performance Supercapacitors, ChemElectroChem, 2015, 2, 1903–1907 CrossRef CAS.
- F. Gong, H. Wang, X. Xu, G. Zhou and Z. S. Wang, In Situ Growth of Co0.85Se and Ni0.85Se on Conductive Substrates as High-Performance Counter Electrodes for Dye-Sensitized Solar Cells, J. Am. Chem. Soc., 2012, 134, 10953–10958 CrossRef CAS PubMed.
- S. C. Tang, S. Vongehr, Y. G. Wang, J. Cui, X. Y. Wang and X. K. Meng, Versatile Synthesis of High Surface Area Multi-Metallic Nanosponges Allowing Control over Nanostructure and Alloying for Catalysis and SERS Detection, J. Mater. Chem. A, 2014, 2, 3648–3660 CAS.
- J. H. Lee, N. Park, B. G. Kim, D. S. Jung, K. Im, J. Hur and J. W. Choi, Restacking-Inhibited 3D Reduced Graphene Oxide for High Performance Supercapacitor Electrodes, ACS Nano, 2013, 7, 9366–9374 CrossRef CAS PubMed.
- L. W. Mi, Q. Ding, H. Sun, W. H. Chen, Y. Y. Zhang, C. T. Liu, H. W. Hou, Z. Zheng and C. Y. Shen, One-pot synthesis and the electrochemical properties of nano-structured nickel selenide materials with hierarchical structure, CrystEngComm, 2013, 15, 2624–2630 RSC.
- B. X. Yuan, W. L. Luan and S. T. Tu, One-step solvothermal synthesis of nickel selenide series: Composition and morphology control, CrystEngComm, 2012, 14, 2145–2151 RSC.
- T. H. Ha, Y. J. Kima and S. H. Park, Complete separation of triangular gold nanoplates through selective precipitation under CTAB micelles in aqueous solution, Chem. Commun., 2010, 46, 3164–3166 RSC.
- M. Matheron, A. Bourgeois, A. Brunet-Bruneau, P. Albouy, J. Biteau, T. Gacoina and J. Boilot, Highly ordered CTAB-templated organosilicate films, J. Mater. Chem., 2005, 15, 4741–4745 RSC.
- D. Ghosh, S. Giri and C. K. Das, Preparation of CTAB-Assisted Hexagonal Platelet Co(OH)2/Graphene Hybrid Composite as Efficient Supercapacitor Electrode Material, ACS Sustainable Chem. Eng., 2013, 1, 1135–1142 CrossRef CAS.
- M. C. López, G. F. Ortiz, P. Lavela, R. Alcántara and J. L. Tirado, Improved Energy Storage Solution Based on Hybrid Oxide Materials, ACS Sustainable Chem. Eng., 2013, 1, 46–56 Search PubMed.
- X. Yu, W. D. Sun and Y. Chu, One-pot solvothermal synthesis and properties of 1D NiSe and NiSe–Ni3S2 alloyed compound nanorod arrays, New J. Chem., 2014, 38, 70–76 RSC.
- Y. F. Xu, M. R. Gao, Y. R. Zheng, J. Jiang and S. H. Yu, Nickel/Nickel(II) Oxide Nanoparticles Anchored onto Cobalt(IV) Diselenide Nanobelts for the Electrochemical Production of Hydrogen, Angew. Chem., Int. Ed., 2013, 52, 8546–8550 CrossRef CAS PubMed.
- L. P. Zhu, W. D. Zhang, H. M. Xiao, Y. Yang and S. Y. Fu, Facile Synthesis of Metallic Co Hierarchical Nanostructured Microspheres by a Simple Solvothermal Process, J. Phys. Chem. C, 2008, 112, 10073–10078 CAS.
- M. Grzelczak, J. Pérez-Juste, B. Rodríguez-González, M. Spasova, I. Barsukov, E. M. Farl and L. M. Liz-Marzán, Pt-Catalyzed Growth of Ni Nanoparticles in Aqueous CTAB Solution, Chem. Mater., 2008, 20, 5399–5405 CrossRef CAS.
- I. Pastoriza-Santos, J. Pérez-Juste and L. M. Liz-Marzán, Silica-Coating and Hydrophobation of CTAB-Stabilized Gold Nanorods, Chem. Mater., 2006, 18, 2465–2467 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra06909f |
| This journal is © The Royal Society of Chemistry 2016 |