A corn stalk-derived porous carbonaceous adsorbent for adsorption of ionic liquids from aqueous solution†
Abstract
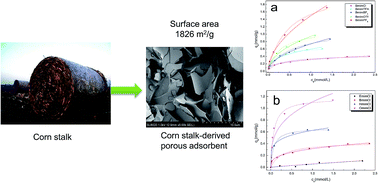
Corn stalks were used to prepare a porous carbonaceous material with a high surface area of 2442 m2 g−1 by the hydrothermal carbonization of corn stalks followed by chemical activation. The prepared corn stalk-derived carbonaceous material (CSCM) was used for the adsorption of ionic liquids (ILs), and showed super performance for the adsorption of ILs from aqueous solution and adsorption capacities of 0.52 and 2.41 mmol g−1 were obtained for [Bmim]Cl and [Bmim][NTf2], respectively. Adsorption was fast that the equilibrium could be reached within about 1 min. The influence of the chemical structure of different ILs on the adsorption onto the CSCM adsorbent was examined where it was revealed that the adsorption capacities of the CSCM for a series of ILs increased with increasing the length of the alkyl chain of the imidazolium cation and the hydrophobicity of the IL anions, followed the sequence of Cl− < [TFA]− < [BF4]− < [OTf]− < [PF6]−. The results of this work provide a facile method for the preparation of a highly efficient adsorbent from renewable agricultural waste that has potential applications in the environmental and chemical fields.

 Please wait while we load your content...
Please wait while we load your content...