Tailor-made poly(L-lactide)/poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds prepared via high-pressure compression molding/salt leaching†
Jin Zhanga,
Shu-Gui Yanga,
Jian-Xun Ding b and
Zhong-Ming Li*a
b and
Zhong-Ming Li*a
aCollege of Polymer Science and Engineering, State Key Laboratory of Polymer Materials Engineering, Sichuan University, Chengdu 610065, Sichuan, P. R. China. E-mail: zmli@scu.edu.cn
bKey Laboratory of Polymer Ecomaterials, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, P. R. China
First published on 9th May 2016
Abstract
A significant challenge in bone tissue engineering is the development of biomimetic scaffolds that can meet the requirements of mechanical and degradation properties at the same time. The composite scaffolds comprising poly(L-lactide) (PLLA), poly(lactide-co-glycolide) (PLGA) and hydroxyapatite (HA) were fabricated by a new method, i.e., high-pressure compression molding plus salt-leaching technique. The scaffolds obtained show an encouraging improvement in the mechanical performance. The compressive modulus reaches up to 4.64 ± 0.2 MPa, comparable to human cancellous bone (2–10 MPa), offering the possibility to develop load-bearing scaffolds. Furthermore, by adjusting the weight ratio of PLLA to PLGA, the degradation rate, hydrophilicity, and mechanical properties of scaffolds can be fine-tuned. The overall characteristics of porous composite scaffolds are definitely optimal when the mass ratio of PLLA/PLGA is 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. Its porosity, contact angle, compressive modulus and weight loss at the 12th week are 81.7%, 53.13°, 4.64 ± 0.2 MPa and 67.21 ± 3.14%, respectively, satisfying the physiological demands to guide tissue regeneration. Scaffolds with the best comprehensive properties are further utilized for in vitro tests. As presented from the excellent spreading and the high proliferation rate of cells, the design of such tailor-made scaffolds as a function of composition is a convenient strategy to address the specific requirements of the tissue to be regenerated.
5. Its porosity, contact angle, compressive modulus and weight loss at the 12th week are 81.7%, 53.13°, 4.64 ± 0.2 MPa and 67.21 ± 3.14%, respectively, satisfying the physiological demands to guide tissue regeneration. Scaffolds with the best comprehensive properties are further utilized for in vitro tests. As presented from the excellent spreading and the high proliferation rate of cells, the design of such tailor-made scaffolds as a function of composition is a convenient strategy to address the specific requirements of the tissue to be regenerated.
1. Introduction
Tissue engineering, as an alternative technique to organ or tissue transplantation, has enormous potential to effectively overcome the problems of immune rejection from the patient as well as the shortage of available donors.1 A key component in tissue engineering is the scaffold that serves as a template to provide structural support for cell interactions and the formation of an extracellular matrix. To allow a high density of seeded cells and to promote the neovascularization when being implanted in vivo, an ideal engineering scaffold should fulfill a number of requirements, including having a highly interconnected pore structure, good biocompatibility and favorable processability.2 In addition to these, one crucial aspect of the tissue engineering is the scaffold biodegradability, which is considered to be at a controlled rate commensurate with tissue remodelling.As a general rule, the degradation rate of scaffolds is mainly determined by the structure, crystallinity and molecular weight of the scaffold material.3 Poly(L-lactide) (PLLA) is the most attractive candidate for scaffold material. It is approved by the Food and Drug Administration (FDA) and can be easily processed into various structures of 3D matrices.4 As shown by the results of in vitro experiments, PLLA scaffold had the highest survival rate of cells compared with other polymers, which was mainly contributed to its high compressive strength and modulus.5 Nevertheless, the chemical structure of PLLA contains an ester bond that is less likely to undergo hydrolysis, so it is a little hydrophobic and degrades slowly (typically 30–50 weeks).6 Poly(lactide-co-glycolide) (PLGA), a new synthetic polypeptide formed by polyglycolic acid and polylactic acid, showing good hydrophilicity, fast degradation rate and being absent of immunogenicity. As a prospective construct material for tissue engineering, the only drawback of PLGA is too flexible, which seriously limits its applications for bone repair materials.7 Given that PLLA and PLGA make a pair of synthetic polypeptide with complementary features, combination of advantages of the two biomaterials will realize some desired properties prior to one of them alone.8 More importantly, the degradation rate of scaffolds could be fine-tuned by adjusting the ratios of PLLA to PLGA. Langer et al. once examined the use of PLLA/PLGA scaffolds for promoting hES cell growth and differentiation and formation of a 3D vessel-like network structure. They claimed that the PLLA/PLGA scaffolds not only possessed a suitable biodegradability, but also could be designed to resist contraction under the compressive stress exerted by the cells.9 Nevertheless, to the best of our knowledge, few investigations once reported systematically on the comprehensive performance of PLLA/PLGA blend for tissue-engineering scaffolds as of yet. Furthermore, fabrication of such scaffolds with tailored compositions, mechanical properties, as well as a proper degradation profile, is essential to mimic the native extracellular matrix and to positively affect the cell behaviour.
Although PLLA or PLGA has been widely accepted as tissue engineering scaffold, they also face the problem of no natural cell recognition sites. As the major inorganic component of bone, hydroxyapatite (HA) has proved capability to promote differentiation of stem cells towards osteoblastic lineage.10 Recently, Gibbons et al. presented a systematic characterisation of bone tissue scaffolds fabricated via 3D printing from HA and poly(vinyl)alcohol (PVOH) composite powders.11 Zhang et al. developed the porous chitosan-HA scaffolds as a mimic of glioblastoma microenvironment ECM.12 In a word, incorporation of HA into PLLA/PLGA blend lays a particular foundation on improving the scaffold bioactivity and osteoconductivity. Additionally, in order to maintain the integrity of the porous structure during the tissue regeneration, scaffolds with enough mechanical strength are actively pursued. Current techniques for attaining porous polyester/HA scaffolds mainly include salt leaching, phase separation, gas foaming, emulsion freeze-drying, and rapid prototype, to name just a few. Nevertheless, the scaffolds fabricated by above-mentioned methods are difficult to engineer clinically useful tissues and organs, which is attributed to the fatal drawbacks of inadequate mechanical properties, especially in the high load sharing situations.13 Compared with these existing methods, the combination of high-pressure compression molding plus salt leaching technique is able to acquire the scaffold with a more compact interpenetrating network structure, an optimized crystalline architecture and an enhanced mechanical property. Based on our previous research work, the storage modulus of PLA/HA scaffolds (87.6 MPa) achieved almost three times higher compared with pure PLA scaffolds, while under low-pressure condition, the increase of modulus caused by HA does not reach 150%. The obvious contrast indicated that HA and high-pressure had a synergistic effect on enhancing mechanical properties of porous scaffolds.14 On this context, such effective method was continued to be utilized for the preparation of PLLA/PLGA/HA composite scaffolds. In the current study, we first discussed the effect of PLLA/PLGA weight ratios on scaffold properties in detail, with respect to porosity, hydrophibility as well as the thermal behaviours, meanwhile the relationship between material composition and macroscopical performance of scaffolds was elucidated. Then, we studied how the degradation rates and mechanical properties of the scaffolds could be fine-tailored by adjusting the ratios, so as to satisfy some specific requirements for the desired tissue. Lastly, we evaluated the biological characteristics of scaffolds according to the results of cellular adhesion and proliferation. Taken together, these findings support our notion that PLLA/PLGA/HA composite scaffolds could be a lead candidate material for tissue engineering.
2. Materials and methods
2.1. Materials
Poly(lactide-co-glycolide) (PLGA, LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GA = 8
GA = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, viscosity-average molecular weight Mη = 10 × 104) and poly(L-lactide) (PLLA, viscosity-average molecular weight Mη = 7.9 × 104, polydispersity Mw/Mη = 2.06) were synthesized and characterized at the Changchun Institute of Applied Chemistry, Chinese Academy of Science, P. R. China.15 Sodium chloride (NaCl) particles with a density of 2.165 g cm−3 were purchased from Chengdu Kelong Chemical Reagent Factory (China) and were used as received. The particle size of NaCl used in current study was sieved in the range from 100 to 200 μm. HA particles with a mean diameter of 6.5 μm were obtained from Chengdu Xinjin Longma Chemical Co., Ltd (China). Such small particle size could guarantee a large surface area, and consequently, might present improved mechanical properties arising from interactions at interfaces.
2, viscosity-average molecular weight Mη = 10 × 104) and poly(L-lactide) (PLLA, viscosity-average molecular weight Mη = 7.9 × 104, polydispersity Mw/Mη = 2.06) were synthesized and characterized at the Changchun Institute of Applied Chemistry, Chinese Academy of Science, P. R. China.15 Sodium chloride (NaCl) particles with a density of 2.165 g cm−3 were purchased from Chengdu Kelong Chemical Reagent Factory (China) and were used as received. The particle size of NaCl used in current study was sieved in the range from 100 to 200 μm. HA particles with a mean diameter of 6.5 μm were obtained from Chengdu Xinjin Longma Chemical Co., Ltd (China). Such small particle size could guarantee a large surface area, and consequently, might present improved mechanical properties arising from interactions at interfaces.
2.2. Preparation of porous PLLA/PLGA/HA scaffolds
Solution coagulation method was utilized to ensure the good distribution of HA in PLLA/PLGA matrix. Taking PLLA/PLGA/HA (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 wt/wt/wt) mixture as an example, the detailed sample preparation was as follows: HA (2.5 g) was added into 50 mL of CH2Cl2, and the solution was subjected to ultrasound and stirred for 40 min to reach a uniform suspension. At the same time, PLLA (5 g) and PLGA (5 g) were completely dissolved in 150 mL of CH2Cl2 by constant stirring. The mixture solution was obtained by adding the CH2Cl2/PLLA/PLGA solution into the CH2Cl2/HA suspension and continuously sonicated for another 40 min. Thereafter, C2H5OH (300 mL) was poured into the mixture solution until no more coagulated material precipitated. Finally, the precipitated mixture was transferred to blowing dryer, left overnight at 55 °C, and dried in a vacuum oven at the same temperature for another 24 h to remove the traces of water. The mixtures with ratio of PLLA/PLGA/HA of 10
2.5 wt/wt/wt) mixture as an example, the detailed sample preparation was as follows: HA (2.5 g) was added into 50 mL of CH2Cl2, and the solution was subjected to ultrasound and stirred for 40 min to reach a uniform suspension. At the same time, PLLA (5 g) and PLGA (5 g) were completely dissolved in 150 mL of CH2Cl2 by constant stirring. The mixture solution was obtained by adding the CH2Cl2/PLLA/PLGA solution into the CH2Cl2/HA suspension and continuously sonicated for another 40 min. Thereafter, C2H5OH (300 mL) was poured into the mixture solution until no more coagulated material precipitated. Finally, the precipitated mixture was transferred to blowing dryer, left overnight at 55 °C, and dried in a vacuum oven at the same temperature for another 24 h to remove the traces of water. The mixtures with ratio of PLLA/PLGA/HA of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5, 7
2.5, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5, 5
2.5, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5, 3
2.5, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 and 0
2.5 and 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 were prepared.
2.5 were prepared.
Porous scaffolds were fabricated by a novel method named high-pressure compression molding/salt leaching techniques. The self-made high-pressure compression molding apparatus is schematically shown in Fig. S1a,† meanwhile the corresponding temperature and pressure protocol are shown in Fig. S1b.† Fig. S2† illustrates the detailed experimental procedures about the fabrication of porous PLLA/PLGA/HA composite scaffolds as described in our previous paper.16
2.3. Morphology observation
Morphology of the porous PLLA/PLGA/HA scaffolds was examined by a field emission scanning electron microscopy (Inspect-F, FEI, Finland), operating in high vacuum and with an accelerating voltage of 20 kV. To expose the interior structure, the specimens were frozen in liquid nitrogen for 20 min, then quickly impact fractured. Prior to SEM examination the freshly broken surfaces were sputtered with gold.2.4. Porosity, connectivity & density of the scaffolds
Quantitative estimation of pore-related parameters of porous PLLA/PLGA/HA scaffolds was performed via gravimetric measurements,17 and the values of porosity, connectivity and density were calculated as follows:| Porosity = (mNaCl/ρNaCl)/(mNaCl/ρNaCl + mPLLA/ρPLLA + mPLGA/ρPLGA + mHA/ρHA) × 100%, | (1) |
| Connectivity = (m0 − m′)/m0NaCl × 100%, | (2) |
| Density = m′/(mNaCl/ρNaCl + mPLLA/ρPLLA + mPLGA/ρPLGA + mHA/ρHA) × 100%, | (3) |
2.5. In vitro degradation of the scaffolds
Degradability of the PLLA/PLGA/HA scaffolds was determined by the mass change of samples after their incubation in PBS solution (pH = 7.4, 37 °C). The PBS solution was replaced every week by a fresh solution and the scaffold degradation studies were carried out for a period of 12 weeks. At the indicated time point, samples were carefully withdrawn from the medium and thoroughly rinsed with distilled water, and dried for 24 h to remove excess water. Measured weights of the samples were normalized against their initials mass to illustrate the fraction of weight loss, which was calculated using the following equation: weight loss = (W0 − Wt)/W0 × 100%, where W0 and Wt are the weights of the scaffolds before and after degradation for a specific time interval, respectively.2.6. Water absorption of the scaffolds
The porous samples were pre-wetted to ensure that water permeated through all the pores of scaffolds. Three dry scaffolds from each group were weighed and then placed in a glass bottle filled with 10 mL water for 24 h. On removal, the scaffolds were carefully wiped with filter paper to remove surface water, followed by measuring the wet weight of the samples. These samples were then dried in vacuum for 12 h to determine the mass of the dried samples. The water absorption was calculated using the following equation:18 water absorption = (Mwet − Mdry)/Mdry × 100%, where Mwet and Mdry are the wet and dry weights of the sample, respectively. Note that the water absorption measured here includes the water absorbed by both the scaffolds and the pores.2.7. Contact angle of the scaffolds
Hydrophilicity of the PLLA/PLGA/HA scaffolds was qualitatively determined by measuring the contact angle of material with distilled water using KRUSS drop shape analyzer (DSA 100). The PLLA/PLGA/HA scaffolds were fixed into the custom made sample holder of the drop shape analyzer. The distilled water was taken in a 2 mL leur lock syringe fitted with blunt edge needle. A single drop of volume ∼2 μL was poured on the scaffolds. The drop shape on the scaffold surface was recorded using the camera attached with system. Initial contact angle values on the scaffolds were measured from each frame of the recorded video files. The contact angle was measured by sessile drop approximation of the inbuilt software in machine.2.8. Thermal behaviors of the scaffolds
Melting behaviors of porous PLLA/PLGA/HA scaffolds with different HA content were studied employing TA Q2000 V7.3 differential scanning calorimeter (DSC). The calibration was performed with indium and all tests were carried out in ultra-pure nitrogen as purge gas. Samples (about 5 mg) enclosed in aluminum pans were heated from 40 to 190 °C at a scanning rate of 10 °C min−1. Melting point temperatures were determined from the melting curves as peak temperatures.2.9. Mechanical properties of the scaffolds
Compressive modulus of the scaffolds was tested using an Instron-4502 machine in ambient atmospheric condition (25 °C and 50% relative humidity, RH), equipped with a 1000 N load cell. A strain rate of 0.5 mm min−1 was used. The specimens were porous cylinders (∼20 mm in diameter and ∼15 mm in thickness) and the modulus values were determined with the initial slope of the stress–strain curve (strain range, 3–8%). At least three samples were tested for each type and the results were averaged.2.10. Cell culture tests of the scaffolds
The PLLA/PLGA/HA composite scaffolds used for cell culture tests were sterilized by immersing in 70% ethanol for 30 min, then rinsed three times with sterile phosphate-buffered saline (PBS) and exposed to UV light for half an hour on each side. Next, scaffolds were transferred into 12-well culture plates and seeded with 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MC3T3-E1 (ATCC) osteoblast-like cells per well. Cells were cultured in α-MEM containing 10% FBS, which was changed every other day after culture. Scaffolds were immobilized in a humidified incubator at 37 °C with 5% of CO2.
000 MC3T3-E1 (ATCC) osteoblast-like cells per well. Cells were cultured in α-MEM containing 10% FBS, which was changed every other day after culture. Scaffolds were immobilized in a humidified incubator at 37 °C with 5% of CO2.
Morphology of the cells cultured on scaffolds was observed using SEM. Cells cultured for 3 days were fixed with 2.5% glutaraldehyde for 2 h at 4 °C. After they had been thoroughly washed with PBS, the samples were dehydrated sequentially through a series of increasing ethanol concentrations (10, 30, 50, 70, 80, 90, 95, and 100%) for 15 min × 2. Finally, scaffolds were dried overnight, coated with Au and examined with SEM. In addition, number of MC3T3-E1 cells was evaluated by adding Cell Counting Kit-8 (CCK-8, Dojindo, Japan) solution to each well. After 1, 3 and 7 days of incubation, numbers of live cells in the samples were counted by measuring the absorbance of the resulting medium at 450 nm through an ELISA reader (Model 550; Bio-Rad, Hercules, CA, U.S.A.). Absorbance at 600 nm was used for baseline correction.
Statistical analysis: all data were expressed as mean ± standard deviation, statistical software SPSS 13.0 was used to analyze the data by one-way analysis of variance; probability value of less than 0.05 was considered significantly different and that below 0.01 or 0.001 was considered highly significantly different.
3. Results and discussion
3.1. Microstructure of PLLA/PLGA/HA scaffolds
After immersing the mixtures in distilled water, space originally occupied by NaCl becomes pores and the PLLA/PLGA/HA phase is still self-supporting, thus forms the final scaffold structure. This treatment ensures the complete connectivity of pores in the scaffold due to the dissolution of continuous NaCl phase, along with a controllable pore diameter which is relied on the size of salt particles. Given that pore structure and pore size have indiscernible dependence on PLLA/PLGA ratios, scaffolds with ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 are taken as an example for morphological analysis. Fig. 1a and b reveal the SEM images of the cross-sections of scaffolds, respectively. The scaffolds possess a well-defined porous structure in three dimensions, with interconnected pores throughout and evenly distributed in PLLA/PLGA/HA skeleton. In addition, the cross-sections of scaffolds exhibit rough pore walls. It was reported that osteoblasts were preferentially adhered to substrates with a certain degree of roughness, for which was conducive to improve the interfacial adhesion between cells and scaffold matrix.19
5 are taken as an example for morphological analysis. Fig. 1a and b reveal the SEM images of the cross-sections of scaffolds, respectively. The scaffolds possess a well-defined porous structure in three dimensions, with interconnected pores throughout and evenly distributed in PLLA/PLGA/HA skeleton. In addition, the cross-sections of scaffolds exhibit rough pore walls. It was reported that osteoblasts were preferentially adhered to substrates with a certain degree of roughness, for which was conducive to improve the interfacial adhesion between cells and scaffold matrix.19
 | ||
| Fig. 1 SEM morphologies (a and b) and bimodal pore size distribution (c) and HA particles dispersion (d) of porous PLLA/PLGA/HA scaffolds. | ||
It can be seen from Fig. 1c that PLLA/PLGA/HA porous scaffolds are obtained with pores of average size between 100 and 150 μm. Zhou et al. once reported that, compared with scaffolds bearing smaller pores (10–50 μm), scaffolds with larger pores (>100 μm) allowed more deposition of collagen and provided enough space for cells vascularization.20 Of great interests, a bimodal porosity characterized by two different pore size ranges is observed in scaffolds. In detail, macropores (10–350 μm in diameter) represent the 74.4% of the total scaffold porosity with an average pore size of about 149.13 μm, while the remaining 25.6% are micropores (1–10 μm in diameter) with an average pore size of 7.65 μm.17 According to the literatures, it is deduced that the interconnected macropores are created by the leaching of continuous NaCl phase, while the closed micropores appeared in the walls of macropores are resulted from some small-sized NaCl droplets which remains as dispersed phase in the skeleton.21 In fact, such bimodal pore structure could be beneficial to the cell–cell communication and nutrient transportation within the scaffolds.22
In most approaches, bioactive materials (such as HA particles) are often deposited onto the surface of prepared porous scaffolds. Weak coating combination of the as-prepared scaffolds is the main disadvantage of these fabrication methods.23 HA particles are partially connected with the polymer matrix and mainly bonded with a weak strength of van der Waals's forces. Differently, combination of high-pressure molding and salt leaching method in current study produces a strict and regular architecture of scaffolds due to the melting-process with high pressure. On this context, HA particles herein were immersed and stayed tightly in PLLA/PLGA matrix by the pressure seepage, guaranteeing the scaffolds a highly improved biocompatibility (Fig. S3 and S4†). Furthermore, as shown in Fig. 1d, a homogenous distribution of HA particles in the scaffolds is revealed and no agglomeration is seen, indicative of an appropriate filler content.
Porosity and pore interconnectivity of scaffolds are critical for cell adhesion and proliferation, along with the diffusion of nutrients and oxygen. Values of porosity, interconnectivity and bulk density of scaffolds for each PLLA/PLGA ratio are measured and the results are shown in Table 1. As expected, at a constant mass fraction of NaCl, porosity of these scaffolds ranges from 81.5% to 82.7%, remaining almost invariable with the changes of PLLA/PLGA ratios. Porosity is often created in solid scaffold by the inclusion of porogen that can be leached away upon placement in an aqueous environment,24 thus mainly dependent on the content of porogen. It is worthy to be noticed that porosity of all scaffolds studied here constantly maintains in high level of above 80%, well meeting the requirements of application in tissue engineering and being able to provide enough space to guide cell proliferation.25 Similarly, variation of PLLA/PLGA ratios has no detectable influence on pore interconnectivity of the formed scaffolds. Its values nearly equalled to 98%, which demonstrates that NaCl within the mixtures is almost fully continuous. The highly interconnected porous materials can be easily seeded with a fluid phase containing cells, for it is desirable for permitting cell infiltration into and throughout the scaffolds. In terms of density obtained by weighing a sample of specific volume, it is just fluctuated around 0.25 g cm−3, nearly the lowest density reported so far for porous PLLA or PLGA scaffolds at such a high porosity and connectivity,10 fully embodying the scaffold prominent superiority of being lightweight. To support all these conclusions, Table 1 is performed in order to confirm the influence of PLLA/PLGA ratios on physical parameters.
| PLLA/PLGA | Porosity (%) | Connectivity (%) | Density (g cm−3) | Tg (°C) | Tm (°C) | Water absorption rate (%) | Maximum load (N) | Compressive modulus (MPa) |
|---|---|---|---|---|---|---|---|---|
| a Note: Porosity, connectivity and the measured density of scaffolds were determined by gravimetric measurements according to eqn (1)–(3), separately. | ||||||||
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
82.7 | 97.7 | 0.250 | 64.1 | 174.4 | 166.7 ± 7.61 | 76.39 ± 2.4 | 6.48 ± 0.55 |
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
83.6 | 98.8 | 0.234 | 60.4 | 170.8 | 220.0 ± 10.12 | 54.08 ± 1.8 | 5.51 ± 0.14 |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
81.7 | 97.3 | 0.262 | 61.0 | 170.3 | 257.1 ± 5.80 | 35.1 ± 2.1 | 4.64 ± 0.20 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
81.5 | 97.1 | 0.264 | 57.7 | 163.3 | 300.0 ± 10.25 | 18.81 ± 1.5 | 3.42 ± 0.12 |
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
81.8 | 97.8 | 0.259 | 56.8 | 152.8 | 357.2 ± 12.40 | 4.86 ± 0.6 | 1.12 ± 0.06 |
3.2. In vitro degradation and hydrophilicity of PLLA/PLGA/HA scaffolds
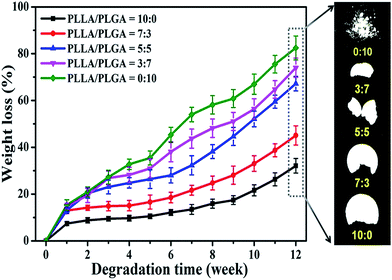
It is required that the scaffold degradation rate needs to be similar to the rate of tissue formation and that the three-dimensional space can be replaced by new tissue. In current study, by varying the PLLA/PLGA weight ratios, we can regulate and control the degradation performance of porous composite scaffolds effectively. Weight loss of the scaffolds in an aqueous environment (PBS) was tested every 7 days in order to track the degradation rate for each scaffold type, as shown in Fig. 2. The higher PLGA content the faster degradation rate of scaffolds. PLGA/HA scaffolds experience 82.47 ± 5.10% weight loss after 12 weeks of incubation, 67.21 ± 3.14% for scaffolds with PLLA/PLGA ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, while the PLLA/HA scaffolds undergo the slowest degradation rate with only 32.11 ± 2.98% weight loss during the same immersion time. Meanwhile, erosion degree of these scaffolds is also obviously different. With the increase of PLGA content, loss of the structural integrity becomes increasingly serious. In contrast to the moderately stable and intact porous structure of PLLA/HA scaffolds, PLGA/HA scaffolds present the collapse of pore structure and finally exist in the appearance of powder. This phenomenon should be explained from two aspects. On one hand, PLGA is more hydrophilic than PLLA component, which can facilitate the contact between scaffolds with PBS and then increase the diffusion of buffered solutions, finally resulting that the polyesters experience hydrolysis reactions more easily. On the other hand, it must be stressed that PLGA belongs to a group of polymers with lower crystallinity compared with PLLA. Tsuji and Miyauchi found that a preferential hydrolysis took place in the free amorphous region rather than in the restricted crystalline region,26 thus a faster degradation of PLGA was revealed.
5, while the PLLA/HA scaffolds undergo the slowest degradation rate with only 32.11 ± 2.98% weight loss during the same immersion time. Meanwhile, erosion degree of these scaffolds is also obviously different. With the increase of PLGA content, loss of the structural integrity becomes increasingly serious. In contrast to the moderately stable and intact porous structure of PLLA/HA scaffolds, PLGA/HA scaffolds present the collapse of pore structure and finally exist in the appearance of powder. This phenomenon should be explained from two aspects. On one hand, PLGA is more hydrophilic than PLLA component, which can facilitate the contact between scaffolds with PBS and then increase the diffusion of buffered solutions, finally resulting that the polyesters experience hydrolysis reactions more easily. On the other hand, it must be stressed that PLGA belongs to a group of polymers with lower crystallinity compared with PLLA. Tsuji and Miyauchi found that a preferential hydrolysis took place in the free amorphous region rather than in the restricted crystalline region,26 thus a faster degradation of PLGA was revealed.
 | ||
| Fig. 2 Trend of the % weight loss of PLLA/PLGA/HA scaffolds with different PLLA/PLGA weight ratios as a function of degradation time. Data were presented as mean ± standard deviation (n = 3). | ||
It is of great interest to notice that changes of foam weight loss are relatively gentle within the first 4 weeks, which have a tendency to exhibit more and more abruptly afterwards. A possible explanation of the observed behaviour stems from the theory of “stage degradation”.27 Scaffolds degradation roughly proceeds in two stages: first is a quasi-stable stage, where water absorption and plasticization occur together and cause a slight increase in the weight loss. Afterwards, along with the extension of immersion time in PBS, disruption of the pore structure and formation of blisters facilitate the degradation and bring about a stage of massive weight loss. In a word, combination of polymers with different degradation kinetics in such “high-pressure compression molding” way, is an effective approach to obtain scaffolds with controlled degradation rates according to some specific biomedical applications.
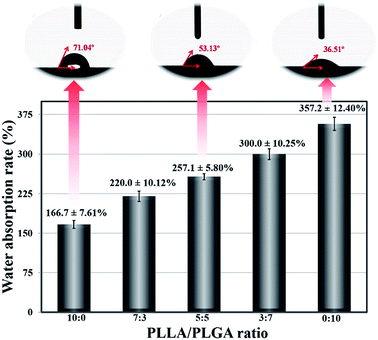
Water absorption is regarded as an important parameter that represents the efficiency of nutrient transfer within the scaffolds. Suitable water absorption ability is extremely necessary to obtain an ideal scaffold, for the high value of it will destroy the scaffolds shape. On the contrary, the terrible absorption will result in the lack of water and finally affect cells normal growth.28 Hydrophilic nature of materials is an important factor that influences the extent of scaffolds swelling capacity. As shown in Fig. 3, water absorption rate of the scaffolds is changed with ratios of PLLA/PLGA; it increases significantly from about 166.7 ± 7.61% to 357.2 ± 12.40% when the ratio varies from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 0
0 to 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. In comparison with PLLA, PLGA is a relatively hydrophilic material and therefore has a very high water uptake.29 Accordingly, the water contact angle measurement is utilized to further understand the hydrophilic and hydrophobic nature of different blend ratios. As shown in Fig. 3, the contact angle values are 71.04, 53.13, and 36.51° for scaffolds with PLLA/PLGA ratio of 10
10. In comparison with PLLA, PLGA is a relatively hydrophilic material and therefore has a very high water uptake.29 Accordingly, the water contact angle measurement is utilized to further understand the hydrophilic and hydrophobic nature of different blend ratios. As shown in Fig. 3, the contact angle values are 71.04, 53.13, and 36.51° for scaffolds with PLLA/PLGA ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 5
0, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, and 0
5, and 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, respectively. Along with the blending of PLGA in PLLA, an obvious decrease in contact angle value is presented, indicative of an improved hydrophilicity of scaffolds surface quality. Additionally, by and large, hydrophilic surfaces would give a contact angle between 0° and 90°.30 Each contact angle detected here is less than 75°, which in turn points towards the excellent hydrophilic behaviour of our fabricated PLLA/PLGA/HA composite scaffolds. Overall, there is a consistent pattern of such increased hydrophilicity with addition of PLGA, as observed from contact angle and water uptake results, which could impart scaffold degradation as well as cell attachment on it.
10, respectively. Along with the blending of PLGA in PLLA, an obvious decrease in contact angle value is presented, indicative of an improved hydrophilicity of scaffolds surface quality. Additionally, by and large, hydrophilic surfaces would give a contact angle between 0° and 90°.30 Each contact angle detected here is less than 75°, which in turn points towards the excellent hydrophilic behaviour of our fabricated PLLA/PLGA/HA composite scaffolds. Overall, there is a consistent pattern of such increased hydrophilicity with addition of PLGA, as observed from contact angle and water uptake results, which could impart scaffold degradation as well as cell attachment on it.
 | ||
| Fig. 3 Water absorption rate and the contact angle images of PLLA/PLGA/HA scaffolds as a function of PLLA/PLGA ratios. Data were presented as mean ± standard deviation (n = 3). | ||
3.3. Thermal and mechanical properties of PLLA/PLGA/HA scaffolds
Variation in the PLLA/PLGA weight ratio has an obvious effect on the thermal behaviour of porous composite scaffolds, including the mesophase formation temperature as well as the melting point. As shown in Fig. 4, a clear mesomorphic phase develops in all samples when the temperature window spanned from 55 to 65 °C, and the mesophase melts and reorganizes into crystallites after the temperature increases to about 90 °C. Along with the incorporation of PLGA into PLLA phase, the mesophase formation temperature drops obviously from 64.1 to 56.8 °C as PLLA/PLGA ratios changes from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 0
0 to 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. According to the literature, PLA mesophase generally forms around the glass transition temperature,31 such evident decrease is attributed to the plasticization effect provoked by PLGA molecules which are in the amorphous state acting as a plasticizer of PLLA.32 In the case of scaffolds with PLLA/PLGA ratio of 5
10. According to the literature, PLA mesophase generally forms around the glass transition temperature,31 such evident decrease is attributed to the plasticization effect provoked by PLGA molecules which are in the amorphous state acting as a plasticizer of PLLA.32 In the case of scaffolds with PLLA/PLGA ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, they present a little higher formation temperature of mesophase (compared with the scaffolds with ratio at 7
5, they present a little higher formation temperature of mesophase (compared with the scaffolds with ratio at 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), which might be ascribed to the higher degree of complex formation and stronger interaction force, for the equal number of functional groups.33 In terms of the melting point, it is remarkable that only a single sharp endothermic melting peak is observed for all the cases, indicating that PLLA and PLGA are quite miscible at least at the given composition. Melting temperature of PLLA/HA scaffold is on the order of 174.4 °C, that is gradually shifted to lower temperatures with increase of PLGA/PLLA ratios, until the PLGA/HA scaffold exhibits a peak located around 152.8 °C. It is because that the melting temperature of PLGA is absolutely lower than that of PLLA in general situation.34
3), which might be ascribed to the higher degree of complex formation and stronger interaction force, for the equal number of functional groups.33 In terms of the melting point, it is remarkable that only a single sharp endothermic melting peak is observed for all the cases, indicating that PLLA and PLGA are quite miscible at least at the given composition. Melting temperature of PLLA/HA scaffold is on the order of 174.4 °C, that is gradually shifted to lower temperatures with increase of PLGA/PLLA ratios, until the PLGA/HA scaffold exhibits a peak located around 152.8 °C. It is because that the melting temperature of PLGA is absolutely lower than that of PLLA in general situation.34
As we all know, scaffolds should have strength as close as possible to that of bone to be repaired or substituted, so that providing enough structural stability to support cell attachment. Compression tests are performed to assess the strength of our fabricated PLLA/PLGA/HA scaffolds. Fig. 5a and b illustrate the maximal load and compressive modulus of scaffolds, respectively, as a function of PLLA/PLGA ratios. With regard to the pure PLA/HA scaffolds, they display strong ability to resist deformation with the maximum load and compressive modulus high up to 76.39 ± 2.4 N and 6.48 ± 0.55 MPa, separately. Compared with the maximal value (21.7 N, 3.36 MPa) of PLA/HA scaffolds once reported with similar porosity and composition, it is at least two times higher, fully revealed an encouraging progress in developing load-bearing scaffolds. Conversely, the pure PLGA/HA scaffolds show the lowest compressive properties (4.86 ± 0.6 N, 1.12 ± 0.06 MPa). Mechanical integrities of composite scaffolds are intermediate between those of the pure components, which exhibit a trend of decline with the increase in mass fraction of PLGA.
 | ||
| Fig. 5 Results of compression tests on composite scaffolds with various ratios of PLLA/PLGA. Data were presented as mean ± standard deviation (n = 3). | ||
In addition, it is interesting to observe that decrease of the mechanical properties is relatively gentle as PLGA content below 50%, which is on the order of 28.4% and 75.9% for scaffolds at PLLA/PLGA ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and 0
5 and 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, separately. As a result, it is reasonable to make a conclusion that incorporation of PLGA into PLLA weakens the carrying capacity of scaffolds. In order to obtain scaffolds with high resistance to external load, choice of PLGA content needs to be less than 50%. One side, mechanical properties of PLLA are more advantageous than those of PLGA, which tends not to deform under the presence of a compressive load for its stiffness.35 On the other side, it was once proposed that in the case of PLGA content less than 50%, the majority phase PLLA was crystalline and the minority PLGA was amorphous, therefore a dense structure and tight connection were provoked.17 On the whole, above data indicates that variation of PLLA/PLGA ratios do show significant influence on modifying mechanical properties of the composite scaffolds at a certain degree.
10, separately. As a result, it is reasonable to make a conclusion that incorporation of PLGA into PLLA weakens the carrying capacity of scaffolds. In order to obtain scaffolds with high resistance to external load, choice of PLGA content needs to be less than 50%. One side, mechanical properties of PLLA are more advantageous than those of PLGA, which tends not to deform under the presence of a compressive load for its stiffness.35 On the other side, it was once proposed that in the case of PLGA content less than 50%, the majority phase PLLA was crystalline and the minority PLGA was amorphous, therefore a dense structure and tight connection were provoked.17 On the whole, above data indicates that variation of PLLA/PLGA ratios do show significant influence on modifying mechanical properties of the composite scaffolds at a certain degree.
As above mentioned, changing PLLA/PLGA weight ratios in composite scaffolds is possible to obtain scaffolds with easily-tunable physical properties adapted to some specials needs of each application. Considering the requirements of scaffolds design on both biodegradability and mechanical performance aspects, the overall properties of porous composite scaffolds are undoubtedly optimal when the weight ratio of PLLA/PLGA is 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. At first, the composite scaffolds experience 67.21 ± 3.14% weight loss after 12 weeks of incubation, which are not only complying with the degradation demands (over 65% in 12 weeks), but also maintaining the structure integrity during the long duration of tissue regeneration. Secondly, compressive modulus of the composite scaffolds reaches up to 4.64 ± 0.2 MPa, a quantity comparable to human cancellous bone (2–10 MPa).36 Such excellent mechanical property could provide a sufficient structural stability to support cell attachment, for which the scaffolds tend not to deform under the presence of a compressive load. Except for these, some other physical characteristics such as porosity of above 80% and contact angle less than 75° further verify the acquisition of a highly interconnected porous structure and a good hydrophilic behavior, which could impart the scaffold a good cell attachment on it. On the basis that the scaffolds are providing a sufficient mechanical strength to ensure the structural integrity, meanwhile satisfying the physiological criteria to guide tissue regeneration. In the following study, cell proliferation assay was performed using the composite scaffolds with PLLA/PLGA weight ratio of 5
5. At first, the composite scaffolds experience 67.21 ± 3.14% weight loss after 12 weeks of incubation, which are not only complying with the degradation demands (over 65% in 12 weeks), but also maintaining the structure integrity during the long duration of tissue regeneration. Secondly, compressive modulus of the composite scaffolds reaches up to 4.64 ± 0.2 MPa, a quantity comparable to human cancellous bone (2–10 MPa).36 Such excellent mechanical property could provide a sufficient structural stability to support cell attachment, for which the scaffolds tend not to deform under the presence of a compressive load. Except for these, some other physical characteristics such as porosity of above 80% and contact angle less than 75° further verify the acquisition of a highly interconnected porous structure and a good hydrophilic behavior, which could impart the scaffold a good cell attachment on it. On the basis that the scaffolds are providing a sufficient mechanical strength to ensure the structural integrity, meanwhile satisfying the physiological criteria to guide tissue regeneration. In the following study, cell proliferation assay was performed using the composite scaffolds with PLLA/PLGA weight ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to evaluate the toxicity of our fabricated resultants.
5 to evaluate the toxicity of our fabricated resultants.
3.4. Proliferation of osteoblast-like cells on PLLA/PLGA/HA scaffolds
To assess whether the PLLA/PLGA/HA composite scaffolds (PLLA/PLGA ratio = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) could provide a suitable biological response for harboring stem cells, osteoblast-like cells (MC3T3-E1) are seeded on the scaffolds and the cell-seeded complex is subject to SEM observation after culturing for 3 days. Similar with the situation in cell culture plate (Fig. 6b), scaffolds (Fig. 6a) are almost completely overgrown by osteoblasts. It is well-known that cells generally have a favorable growth state in plastic plate; result comparable to the control sample fully verifies that our scaffolds possess high cellular compatibility and then can effectively accelerate the cells' infiltration. While, different from the cells with flattened and rich filopodia on the cell culture plate, MC3T3-E1 cells proliferated on the composite scaffolds exhibit the other state of round shape. Some of them adhere on the macropore surface, others penetrate well into the scaffolds and span the pores in a 3D fashion. This phenomenon has already been verified by some other literature,37 which is mainly because of a larger pore size compared with the cells dimension. Additionally, it is revealed that a great deal of fibrous extracellular matrix (ECM) was secreted surrounding the cells due to the metabolism effect, which exhibited the continuous shape and covered on the surfaces of the pores (Fig. 6a).
5) could provide a suitable biological response for harboring stem cells, osteoblast-like cells (MC3T3-E1) are seeded on the scaffolds and the cell-seeded complex is subject to SEM observation after culturing for 3 days. Similar with the situation in cell culture plate (Fig. 6b), scaffolds (Fig. 6a) are almost completely overgrown by osteoblasts. It is well-known that cells generally have a favorable growth state in plastic plate; result comparable to the control sample fully verifies that our scaffolds possess high cellular compatibility and then can effectively accelerate the cells' infiltration. While, different from the cells with flattened and rich filopodia on the cell culture plate, MC3T3-E1 cells proliferated on the composite scaffolds exhibit the other state of round shape. Some of them adhere on the macropore surface, others penetrate well into the scaffolds and span the pores in a 3D fashion. This phenomenon has already been verified by some other literature,37 which is mainly because of a larger pore size compared with the cells dimension. Additionally, it is revealed that a great deal of fibrous extracellular matrix (ECM) was secreted surrounding the cells due to the metabolism effect, which exhibited the continuous shape and covered on the surfaces of the pores (Fig. 6a).
 | ||
Fig. 6 SEM micrographs of (a) PLLA/PLGA/HA scaffolds with PLLA/PLGA ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and (b) cell culture plate after culturing osteoblast-like cells (MC3T3-E1) for 3 days. 5 and (b) cell culture plate after culturing osteoblast-like cells (MC3T3-E1) for 3 days. | ||
Fig. 7 shows the relative cellular proliferation after cultured on cell culture plate and high-pressure fabricated PLLA/PLGA/HA composite scaffolds with PLLA/PLGA ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 for 1, 3 and 7 days. In line with the result of cells morphology, cells number of the high-pressure compression molded PLLA/PLGA/HA composite scaffolds is matched to that of the plastic plate at all culturing time points, further suggesting that our fabricated scaffolds possess a great potential to support cellular proliferation. The morphology and proliferation of cells on the high-pressure compression molded scaffolds are further compared with those on the conventional compression molded scaffolds (as shown in Fig. S5†).
5 for 1, 3 and 7 days. In line with the result of cells morphology, cells number of the high-pressure compression molded PLLA/PLGA/HA composite scaffolds is matched to that of the plastic plate at all culturing time points, further suggesting that our fabricated scaffolds possess a great potential to support cellular proliferation. The morphology and proliferation of cells on the high-pressure compression molded scaffolds are further compared with those on the conventional compression molded scaffolds (as shown in Fig. S5†).
4. Conclusions
High-pressure compression molding plus salt-leaching techniques were utilized to fabricate the composite scaffolds comprising poly(L-lactide) (PLLA), poly(lactide-co-glycolide) (PLGA) and hydroxyapatite (HA). By adjusting the weight ratios of PLLA to PLGA, degradation rate and the mechanical properties of scaffolds can be fine-tuned to satisfy some specific biomedical applications. The optimized scaffold composition was studied in relation to the porosity, pore morphology, degradation behaviour, hydrophilicity, thermal property as well as the mechanical performance of scaffolds. At PLLA/PLGA ratio is 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, porosity, compressive modulus, contact angle and weight loss at 12th week of scaffolds are 81.7%, 4.64 ± 0.2 MPa, 53.13° and 67.21 ± 3.14%, respectively, not only producing interconnected porous scaffolds with a sufficient mechanical strength to ensure the structural integrity, but also meeting the physiological demands to guide tissue regeneration. More importantly, compressive modulus of our fabricated scaffolds can reach up to 4.64 ± 0.2 MPa, a quantity comparable to human cancellous bone (2–10 MPa), fully revealed an encouraging progress in developing load-bearing scaffolds. Additionally, as revealed from the morphology and number of the cells cultured in scaffolds, our resultant PLLA/PLGA/HA scaffolds with different PLLA/PLGA ratios are well-suited candidates for the design of tailor-made matrices in tissue engineering.
5, porosity, compressive modulus, contact angle and weight loss at 12th week of scaffolds are 81.7%, 4.64 ± 0.2 MPa, 53.13° and 67.21 ± 3.14%, respectively, not only producing interconnected porous scaffolds with a sufficient mechanical strength to ensure the structural integrity, but also meeting the physiological demands to guide tissue regeneration. More importantly, compressive modulus of our fabricated scaffolds can reach up to 4.64 ± 0.2 MPa, a quantity comparable to human cancellous bone (2–10 MPa), fully revealed an encouraging progress in developing load-bearing scaffolds. Additionally, as revealed from the morphology and number of the cells cultured in scaffolds, our resultant PLLA/PLGA/HA scaffolds with different PLLA/PLGA ratios are well-suited candidates for the design of tailor-made matrices in tissue engineering.
Acknowledgements
The authors gratefully acknowledge the financial support from the Program of National Natural Science Foundation of China (Grants No. 51303174, 51273196, 51321062, 51533004) and the Innovation Team Program of Science & Technology Department of Sichuan Province (grant 2014TD0002).Notes and references
- K. C. Shin, B. S. Kim, J. H. Kim, T. G. Park, J. D. Nam and D. S. Lee, Polymer, 2005, 46, 3801–3808 CrossRef CAS.
- C. R. Kothapalli, M. T. Shaw and M. Wei, Acta Biomater., 2005, 1, 653–662 CrossRef PubMed.
- S. Liao, F. Watari, Y. Zhu, M. Uo, T. Akasaka, W. Wang, G. Xu and F. Cui, Dent. Mater., 2007, 23, 1120–1128 CrossRef CAS PubMed.
- J. Han, P. Lazarovici, C. Pomerantz, X. Chen, Y. Wei and P. I. Lelkes, Biomacromolecules, 2010, 12, 399–408 CrossRef PubMed.
- A. G. Mikos and J. S. Temenoff, Electron. J. Biotechnol., 2000, 3, 23–24 Search PubMed.
- G. Pan, S. Liu, X. Zhao, J. Zhao, C. Fan and W. Cui, Biomaterials, 2015, 53, 202–210 CrossRef CAS PubMed.
- T. Endogan Tanir, V. Hasirci and N. Hasirci, Polym. Compos., 2015, 36, 1917–1930 CrossRef CAS.
- S. K. Sahoo, A. K. Panda and V. Labhasetwar, Biomacromolecules, 2005, 6, 1132–1139 CrossRef CAS PubMed.
- S. Levenberg, N. F. Huang, E. Lavik, A. B. Rogers, J. Itskovitz-Eldor and R. Langer, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 12741–12746 CrossRef CAS PubMed.
- C. Guo, X. Guo, N. Cai and Y. Dong, Mater. Lett., 2012, 74, 197–199 CrossRef CAS.
- S. C. Cox, J. A. Thornby, G. J. Gibbons, M. A. Williams and K. K. Mallick, Mater. Sci. Eng., C, 2015, 47, 237–247 CrossRef CAS PubMed.
- S. J. Florczyk, K. Wang, S. Jana, D. L. Wood, S. K. Sytsma, J. G. Sham, F. M. Kievit and M. Zhang, Biomaterials, 2013, 34, 10143–10150 CrossRef CAS PubMed.
- P. X. Ma and J. W. Choi, Tissue Eng., 2001, 7, 23–33 CrossRef CAS PubMed.
- J. Zhang, H.-M. Yin, B. S. Hsiao, G.-J. Zhong and Z.-M. Li, J. Mater. Sci., 2014, 49, 1648–1658 CrossRef CAS.
- Z. Song, J. Yin, K. Luo, Y. Zheng, Y. Yang, Q. Li, S. Yan and X. Chen, Macromol. Biosci., 2009, 9, 268–278 CrossRef CAS PubMed.
- L. R. Rodrigues, M. A. dÁvila, F. J. M. Monteiro and C. A. d. C. Zavaglia, Mater. Res., 2012, 15, 974–980 CrossRef CAS.
- V. Guarino, F. Causa, P. Taddei, M. di Foggia, G. Ciapetti, D. Martini, C. Fagnano, N. Baldini and L. Ambrosio, Biomaterials, 2008, 29, 3662–3670 CrossRef CAS PubMed.
- L. Zheng, F. Yang, H. Shen, X. Hu, C. Mochizuki, M. Sato, S. Wang and Y. Zhang, Biomaterials, 2011, 32, 7053–7059 CrossRef CAS PubMed.
- J. Ferdous, V. B. Kolachalama and T. Shazly, Acta Biomater., 2012, 40, 955–965 Search PubMed.
- A. Obata, T. Hotta, T. Wakita, Y. Ota and T. Kasuga, Acta Biomater., 2010, 6, 1248–1257 CrossRef CAS PubMed.
- Z. Yuan and B. D. Favis, AIChE J., 2005, 51, 271–280 CrossRef CAS.
- S.-M. Lien, L.-Y. Ko and T.-J. Huang, Acta Biomater., 2009, 5, 670–679 CrossRef CAS PubMed.
- Y. Chen, A. F. Mak, M. Wang and J. Li, J. Biomed. Mater. Res., Part B, 2006, 77, 315–322 CrossRef PubMed.
- Y. Kang, G. Yin, Q. Yuan, Y. Yao, Z. Huang, X. Liao, B. Yang, L. Liao and H. Wang, Mater. Lett., 2008, 62, 2029–2032 CrossRef CAS.
- Y. T. Shieh and G. L. Liu, J. Polym. Sci., Part B: Polym. Phys., 2007, 45, 466–474 CrossRef CAS.
- Y. Kikkawa, H. Abe, T. Iwata, Y. Inoue and Y. Doi, Biomacromolecules, 2002, 3, 350–356 CrossRef CAS PubMed.
- J. Blaker, S. Nazhat, V. Maquet and A. Boccaccini, Acta Biomater., 2011, 7, 829–840 CrossRef CAS PubMed.
- S. Yan, K. Zhang, Z. Liu, X. Zhang, L. Gan, B. Cao, X. Chen, L. Cui and J. Yin, J. Mater. Chem. B, 2013, 1, 1541–1551 RSC.
- D. Li, H. Sun, X. Hu, Y. Lin and B. Xu, Mater. Technol., 2013, 28, 316–323 CrossRef CAS.
- X. Wei, B. Zhao, X.-M. Li, Z. Wang, B.-Q. He, T. He and B. Jiang, J. Membr. Sci., 2012, 407, 164–175 CrossRef.
- S. S. Jawalkar and T. M. Aminabhavi, Polymer, 2006, 47, 8061–8071 CrossRef CAS.
- D. Velasco, L. Benito, M. Fernández-Gutiérrez, J. San Román and C. Elvira, J. Supercrit. Fluids, 2010, 54, 335–341 CrossRef CAS.
- B. Cao, J. Yin, S. Yan, L. Cui, X. Chen and Y. Xie, Macromol. Biosci., 2011, 11, 427–434 CrossRef CAS PubMed.
- P. S. Wolfe, S. A. Sell and G. L. Bowlin, Tissue Eng., 2011, 41–67 Search PubMed.
- M.-W. Sa and J. Y. Kim, Int. J. Precis. Eng. Manuf., 2013, 14, 649–655 CrossRef.
- H. R. R. Ramay and M. Zhang, Biomaterials, 2004, 25, 5171–5180 CrossRef CAS PubMed.
- J. Yang, G. Shi, J. Bei, S. Wang, Y. Cao, Q. Shang, G. Yang and W. Wang, J. Biomed. Mater. Res., 2002, 62, 438–446 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra06906a |
| This journal is © The Royal Society of Chemistry 2016 |


