CO2 chemisorption in Li2CuO2 microstructurally modified by ball milling: study performed with different physicochemical CO2 capture conditions†
Hugo A. Lara-Garcíaa,
Margarita J. Ramírez-Morenoa,
José Ortiz-Landerosb and
Heriberto Pfeiffer*a
aInstituto de Investigaciones en Materiales, Universidad Nacional Autónoma de México, Circuito exterior s/n, Cd. Universitaria, Del. Coyoacán, CP 04510, Ciudad de México, Mexico. E-mail: pfeiffer@iim.unam.mx; Tel: +52 55 5622 4627
bDepartamento de Ingeniería en Metalurgia y Materiales, Escuela Superior de Ingeniería Química e Industrias Extractivas, Instituto Politécnico Nacional, Av. Instituto Politécnico Nacional s/n, CP 07738, Ciudad de México, Mexico
First published on 2nd June 2016
Abstract
Lithium cuprate (Li2CuO2) was obtained by a solid state reaction and a subsequent ball milling process; then, the samples were characterized structurally and microstructurally. Additionally, both the Li2CuO2 ball milled and the solid state samples, for comparison purposes, were tested in the CO2 chemisorption process at moderate and low temperatures under different reaction conditions: (i) at moderate CO2 pressure and (ii) in the presence of water vapor. In both cases, the textural and microstructural properties of the ball milled Li2CuO2 samples showed excellent CO2 chemisorption properties which are significantly enhanced due to CO2 pressure effects or the presence of water vapor. All these results were attributed to the textural and morphological changes evidenced in the samples. The observed surface area increments show preponderant effects during CO2 chemisorption at low and moderate temperatures.
1. Introduction
Recently, the concentration of CO2 in the atmosphere reached its highest level in history, which is causing serious environmental problems. The principal issue is an increment in the global surface temperature, which is caused by the greenhouse effect. In order to reduce the concentrations of greenhouse gases, several strategies have been proposed. Among the different solutions, CO2 capture-storage is a possible way to reduce the concentration of these gases.In this regard, different materials have been studied as CO2 captors. These materials should have certain properties in order to be considered ideal captors, such as large capacity of capture, adequate kinetics, thermal stability, cyclability and capability to operate in a wide thermal range. Some of the most studied materials are amines, hydrotalcites, zeolites, metal salts, carbon-based materials, metal–organic frameworks (MOFs) and metal oxides, among others. These materials can be separated by their exhibited temperature CO2 capture ranges as low (<100 °C), moderate (100 to 400 °C) or high (>400 °C) temperature captors.1–5
Among the metal oxides, alkaline ceramics are one of the most studied materials for CO2 capture. These materials present some of the previously mentioned ideal properties. Among the most analyzed alkaline ceramics are lithium and sodium zirconates,6–10 lithium silicates,11–16 and sodium titanate.17,18 Literature reports show that these materials are able to trap CO2 at high temperatures (>400 °C), although it has been demonstrated that certain alkaline ceramics can also chemisorb CO2 at low temperatures in the presence of water vapor. Under these physicochemical conditions, water vapor works as an active catalytic species, enhancing the carbonation process.19–24
Alkaline ceramics usually present low surface areas, which limits the CO2 chemisorption process. In order to improve this microstructural property, some alkaline ceramics have been synthesized by different methods.25–31 For example, lithium orthosilicate (Li4SiO4) and metasilicate (Li2SiO3) have been synthesized by ball milling30 and hydrothermal26 techniques, respectively. In both cases, the surface areas were increased by one order of magnitude, improving the CO2 chemisorption process. In a different work, macroporous Li4SiO4 was prepared by a simple solid-state transformation method using LiOH and fumed silica.37 As expected, the macroporous microstructure improved the CO2 capture kinetics and efficiency.
Analogously, it was recently proved that the variation of pressure in a closed system enhances CO2 capture in lithium oxosilicate (Li8SiO6) at moderate temperatures (T < 400 °C). Meanwhile, this material reacts with less than 1 mmol of CO2 per gram of ceramic at atmospheric conditions, while at pressures higher than atmospheric (up to 1000 kPa) the CO2 captured was 6.8 mmol g−1.32
On the other hand, lithium cuprate (Li2CuO2) presents very good CO2 chemisorption properties. Li2CuO2 is able to trap CO2 in a wide range of temperatures (40 to 750 °C).19,33–36 Li2CuO2 chemisorbs CO2 under different thermal conditions, including high and at low temperatures, with the addition of water vapor in the second case. Theoretically, the maximum CO2 chemisorption capacity of Li2CuO2 is 9.11 mmol of CO2 per gram of Li2CuO2 (0.401 gCO2/gLi2CuO2). Therefore, the aim of this work was to analyze the microstructural characteristics of Li2CuO2 that has been mechanically modified via ball milling and to evaluate the CO2 chemisorption process. This study was performed under two different physicochemical conditions: i.e., CO2 capture at moderate CO2 pressure and in the presence of water vapor.
2. Experimental section
Lithium cuprate was synthesized via a solid-state reaction. The precursors, lithium oxide (Li2O, Aldrich) and copper oxide (CuO, Acros Organics), were mechanically mixed and heated at 800 °C for 6 h in air. Afterwards, the powders were processed by dry ball milling using a Chemplex shaker ball mill (Spectro Mill II). The milling time was varied between 10 and 60 min, using air atmosphere. After that, the samples were sealed and stored in a moisture and CO2 free container. The pristine and milled Li2CuO2 samples were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM) and N2 adsorption–desorption measurements.For the XRD analysis, a diffractometer (D5000, Siemens) coupled to a cobalt X-ray tube was used. The crystalline phases were identified using the Joint Committee Powder Diffraction Standards (JCPDS) database. Then, the morphological characteristics of the Li2CuO2 samples were determined by SEM, which was performed using a JEOL JMS-7600F microscope. Finally, N2 adsorption–desorption measurements were conducted using Bel-Japan Minisorp II equipment to determine the samples surface areas by using the BET model.
The CO2 capture was evaluated by thermogravimetry. Initially, dynamic temperature experiments were performed in a TA Instruments Q500HR thermobalance. In this case, the samples were heated at 5 °C min−1, from 30 °C to 850 °C, in a CO2 saturated atmosphere (Praxair, grade 3.0). Then, the CO2 sorption was evaluated in the presence of water vapor; in this case, a Q5000SA thermobalance (from TA Instruments) was used. Here, different dynamic and isothermal experiments were performed with varying temperature and relative humidity (RH). These experiments were performed using distilled water and two different flow gases: nitrogen (N2, Praxair grade 4.8) or carbon dioxide (CO2, Praxair grade 3.0), following the methodology described in a previous paper.22
To evaluate the CO2 sorption at moderate pressure on lithium cuprate, a volumetric Belsorp-HP instrument from Bel-Japan and a pressure differential scanning calorimeter (P-DSC, Instrument Specialists Incorporated) were used. In the Belsorp-HP equipment, dynamic pressure experiments were performed at different temperatures (between 30 and 350 °C). The samples were pressured from 5.0 Pa to 2.5 MPa in a CO2 atmosphere. Additionally, different dynamic temperature experiments were carried out using pressure DSC equipment at 2.5 MPa using two different atmospheres: N2 and CO2. The samples were heated from room temperature to 410 °C at 10 °C min−1. It should be noted that during the P-DSC experiments, the pressure varied slightly as a function of temperature.
Finally, after the different CO2 capture experiments, the sample products were re-characterized. These powders were analyzed using XRD, decomposition thermogravimetric analysis (TGA), N2 adsorption–desorption measurements and scanning electron microscopy (SEM). XRD, N2 adsorption and SEM characterizations were performed as described above. Decomposition TG measurements were performed under a nitrogen atmosphere using the same Q500HR TA Instruments equipment, from 30 to 950 °C at 5 °C min−1. These experiments were performed to quantify the CO2 captured during the CO2–H2O experiments.
3. Results and discussion
Lithium cuprate was microstructurally modified by a ball milling process for different times, between 10 and 60 min. All these samples were characterized by XRD (Fig. 1) in addition to the pristine Li2CuO2 solid state sample. As can be seen, the Li2CuO2 structure was preserved in all the ball milled samples; however, the corresponding diffraction peaks became broader than those observed for the solid-state sample. The crystal sizes of the ball milled samples were estimated using the Scherrer equation, varying from 112.4 ± 10 to 80.2 ± 8 Å after 10 and 60 min of milling, respectively. The crystal size of the solid state sample was measured; however, it was larger than 500 Å, which exceeds the detection limit. In any case, it is evident that the ball milling process decreased the Li2CuO2 crystallite size. Moreover, it should be noted that no secondary phases were detected in any of the ball milled samples within the XRD detection limits, which implies that the Li2CuO2 is chemically stable after the mechanical procedure. | ||
| Fig. 1 XRD patterns of Li2CuO2 synthesized by solid state reaction and Li2CuO2 microstructurally modified by ball milling for different milling times. | ||
N2 adsorption–desorption and SEM experiments were performed to determine the microstructural characteristics of the Li2CuO2 milled samples. Fig. 2 shows the N2 adsorption–desorption isotherms of Li2CuO2 before (solid-state sample) and after the ball milling processes. According to the IUPAC classification,38 all the samples presented type IIb isotherms with narrow H3 hysteresis loops, corresponding to non-porous or macroporous materials, while the hysteresis loop can be associated with non-rigid aggregates.38,39 The BET surface area was determined for all these samples; while the solid state sample presented values of 0.3 m2 g−1, all the milled samples presented surface areas greater than 2.8 m2 g−1. This means that surface area increased by one order of magnitude after the ball milling process. Additionally, it must be pointed out the greatest surface area was obtained after the shortest ball milling process (10 min); this was equal to 4.9 m2 g−1. After that, the surface areas tended to decrease, from 4.9 to 2.8 m2 g−1 when the milling times were varied from 10 to 60 min, respectively. It seems that a longer milling process induces an incipient aggregation of the ultimate particles due to the high energy of the process.
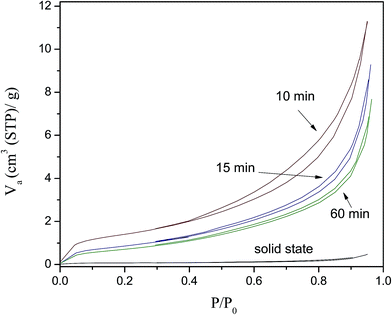 | ||
| Fig. 2 N2 adsorption–desorption isotherms of pristine Li2CuO2 and after different milling times of the dry ball milling process. | ||
Fig. 3 shows the morphology of the Li2CuO2 samples before (A and B) and after (C and D) 10 min of ball milling. Although both samples presented similar textural properties, the solid-state Li2CuO2 sample appears to present larger particles than the ball milled sample. While the solid-state particle size was around 10 to 20 μm, the ball milled sample image shows the presence of particles with different sizes: 5 to 10 μm, and another fraction of particles of around 0.3 to 0.5 μm. These SEM results are in good agreement with the other microstructural analysis (N2 adsorption). Hence, these Li2CuO2 microstructural variations are expected to induce important improvements in the CO2 chemisorption process, as has been reported in previous papers for other lithium and sodium ceramics,25–31,37 where the CO2 chemisorption is importantly related to the particle size and surface area.
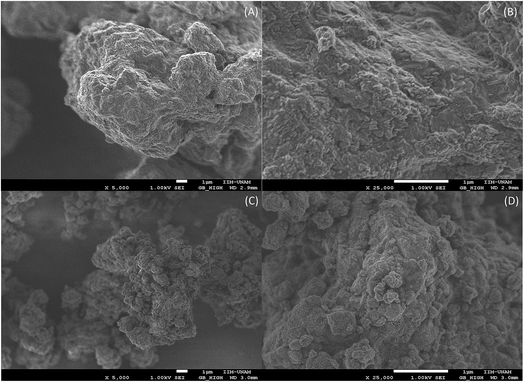 | ||
| Fig. 3 Secondary electron images of the Li2CuO2 solid state sample (A and B) and after 10 minutes of dry ball milling (C and D). | ||
After the structural and microstructural characterization of the Li2CuO2 ball milled sample, the CO2 chemisorption was evaluated dynamically as a function of temperature. According to the literature, Li2CuO2 reacts with CO2 as shown in reaction (1). Here, the Li2CuO2 sample ball milled for 10 min was selected for all the CO2 capture experiments, as it presented the highest surface area. In this case, Fig. 4 shows the thermograms of the Li2CuO2 sample prepared by the solid-state reaction and the same sample after the ball milling process in a CO2 saturated atmosphere.
| Li2CuO2 + CO2 ↔ Li2CO3 + CuO | (1) |
 | ||
| Fig. 4 Dynamic thermogravimetric analysis of solid state Li2CuO2 and Li2CuO2 dry ball milled for 10 minutes, under a saturated atmosphere of CO2 (60 mL min−1). | ||
The Li2CuO2 solid state sample exhibited typical carbonation behavior, which is in good agreement with previous reports wherein dynamic thermogravimetric studies were performed.33–36 This sample presented a double chemisorption process, where the superficial and bulk processes were performed between 250 to 450 and 610 to 740 °C, respectively. The maximum CO2 chemisorption was obtained and stabilized at 850 °C, being equal to 34 wt%. This sample did not present any desorption process; nevertheless, it should be pointed out that this is a dynamic process in a CO2 saturated atmosphere. In contrast, the Li2CuO2 ball milled sample showed a significant improvement in its CO2 chemisorption at moderate temperatures (100 to 400 °C).
Although the maximum superficial chemisorption temperature was not shifted (∼400 °C), the CO2 chemisorption temperature range was importantly broadened to lower temperatures; the range of the ball milled sample began at around 80 °C, while the solid state sample only began to chemisorb CO2 superficially at 250 °C.
The maximum increase in mass due to superficial chemisorption was equal to 17.3 wt%, which corresponds to more than three times the amount of CO2 chemisorbed in the solid-state sample (4.7 wt%). On the other hand, at high temperatures, the CO2 chemisorption observed on the ball milled sample was not as high as that on the Li2CuO2 solid state sample. In this case, the maximum CO2 chemisorbed was 28.3 wt%, and the thermogram shows the beginning of a CO2 desorption process. This may have occurred due to different solid–gas interface saturations; these are modified by the increment of the surface area, which consequently modifies the CO2 chemisorption–desorption equilibrium. Similar results have been obtained in other lithium ceramics once their initial surface areas are improved.26–31
Based on these results, it is clearly evident that the microstructural changes have important influence at low and moderate temperatures during the CO2 capture process in Li2CuO2. Therefore, two different sets of experiments were performed: (1) CO2 chemisorption at low-moderate temperatures (30 to 350 °C) under different pressures (up to 2500 kPa) and (2) CO2 chemisorption at low temperatures (30 to 80 °C) in the presence of water vapor. These physicochemical variations were selected because it has been reported that alkaline ceramics improve their CO2 chemisorption when the CO2 pressure is increased32 and in the presence of water vapor,19–24 both in the same temperature range; under these conditions, the Li2CuO2 ball milled sample presented important improvements in comparison to the solid-state sample.
Fig. 5 shows the CO2 chemisorption isotherms on the Li2CuO2 ball milled sample for 10 min at different temperatures (30 to 350 °C) and moderate pressures (5 to 2500 kPa). Between 30 and 150 °C, the CO2 chemisorption was very low (≤0.2 mmol g−1). However, at 200 °C, the CO2 chemisorption increased exponentially up to 2 mmol g−1, while the pressure only reached 100 kPa. At higher pressures, the CO2 capture did not seem to increase. When the temperature was increased between 250 and 350 °C, the CO2 capture presented similar exponential behavior, trapping up to 5.7 mmol g−1 of CO2 at 350 °C. For comparison purposes, the Li2CuO2 solid-state sample was evaluated at 350 °C. This sample presented the same exponential behavior; however, the CO2 trapped was only 1.7 mmol g−1, at 600 kPa. Therefore, if the trapped CO2 is compared between the Li2CuO2 prepared by solid-state and ball milled processes, it is clearly evident that the microstructurally modified Li2CuO2 has significantly better CO2 capture properties, as it captured more than three times the CO2 captured by the solid state sample. Additionally, the amount of CO2 captured by the Li2CuO2 ball milled sample under these temperature and pressure conditions in a closed system is substantially higher than those observed in previous studies of Li2CuO2 at atmospheric pressures between 30 and 350 °C,33–36 where the CO2 chemical capture was not higher than 0.5 mmol g−1. The highest chemisorption under these physicochemical conditions was achieved at 350 °C with the ball milled sample, corresponding to 63% of the maximum theoretical CO2 chemisorption capacity for lithium cuprate. It should be noted that most of the chemisorption process occurred when the pressure reached 100 kPa; at higher pressures, the chemisorption did not increase significantly. Considering that in this temperature range the diffusion processes are not activated, these results strongly suggest that ball milled Li2CuO2 sample trapped more CO2 than the solid-state sample at moderate temperatures because of the surface area and because the chemisorption process occurs in a close system.
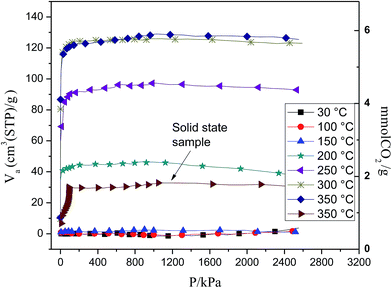 | ||
| Fig. 5 Moderate-pressure CO2 sorption isotherms at different temperatures of solid state Li2CuO2 and Li2CuO2 dry ball milled for 10 minutes. | ||
In order to confirm that CO2 was chemically trapped, the isothermal products were re-characterized using X-ray diffraction (structurally), N2 adsorption–desorption, and scanning electron microscopy (microstructurally). Fig. 6 shows the XRD patterns of all the isothermal products. The sample treated at 30 °C did not evidence any kind of structural change, as only the Li2CuO2 diffraction peaks were depicted. Perhaps the most evident change corresponds to an incipient recrystallization process in comparison to the Li2CuO2 XRD pattern obtained after 10 min of ball milling. At higher temperatures, other crystalline phases were detected: copper oxide (CuO) and lithium carbonate (Li2CO3), as expected. The presence of these phases confirmed that CO2 was chemically trapped. In other words, CO2 was chemisorbed according to reaction (1).
After the XRD characterization, the isotherm products were microstructurally evaluated by N2 adsorption–desorption (see complementary data) and scanning electron microscopy (Fig. 7). The N2 adsorption–desorption results showed type-II isotherms according to the IUPAC classification,38 without any kind of hysteresis loop. Moreover, in all the isothermal products, the surface area decreased after the CO2 moderate pressure capture process, from 4.9 m2 g−1 (Li2CuO2 ball-milled sample) to 1.9 to 2.7 m2 g−1 in the isothermal products. These results provide evidence of densification, which should be attributed to both the carbonation and moderate pressure effects. To complement the CO2 chemisorption analysis on Li2CuO2 at moderate pressure, the microstructural characterization was completed using SEM. Fig. 7 shows the secondary electron images of the ball milled Li2CuO2 sample after the moderate pressure CO2 capture process. All the Li2CuO2 product particles presented a dense polyhedral morphology, increasing the particle size from 10 to 40 μm as a function of temperature. This means that particle size increased up to four times after the CO2 carbonation, due to the moderate pressure effect. Additionally, these large particles presented a well-defined roughness, which seems to be produced by the sintering process and the coalescence of very tiny particles. The backscatter electron image of these particles evidenced the presence of two different phases (see the inset in Fig. 7D), Li2CO3 and CuO, which corresponds to the dark and light phases, respectively.19 For comparison purposes, Fig. 8 shows the morphology evolution of the solid-state Li2CuO2 sample after the CO2 capture process at moderate pressure and 350 °C. In this case, the final particles grew to 50 μm on average, and the surface showed high densification but without any evident roughness. As was previously shown, the Li2CuO2 solid-state particles were much larger; however, their CO2 capture was three times smaller than that observed for the ball-milled Li2CuO2 sample. Therefore, the final particle size corresponds to the initial large particles and the Li2CO3–CuO external shell may not have the same texture observed in the case of the ball-milled sample.
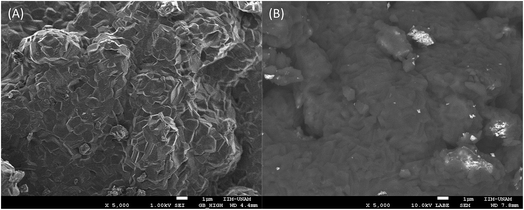 | ||
| Fig. 8 Secondary (A) and backscattered (B) electron images of the Li2CuO2 solid state sample treated at 350 °C under moderate CO2 pressure. | ||
In addition, moderate pressure DSC experiments were performed for the Li2CuO2 solid-state and ball milled samples in CO2 and N2 atmospheres at a pressure of 2.5 MPa (Fig. 9). Li2CuO2 presented different P-DSC thermograms, depending on atmosphere (N2 or CO2). Although both atmospheres induced an endothermic trend, in the solid state case, the Li2CuO2–CO2 system presented a wide exothermic peak between 250 and 400 °C. This exothermic peak must be related to the CO2 chemisorption process. In fact, when the Li2CuO2 ball milled sample was tested in 2.5 MPa of CO2, the exothermic peak was wider than in the solid-state sample. In this case, the CO2 chemisorption was evidenced by the exothermic behavior to occur from 70 to 400 °C.
 | ||
| Fig. 9 DSC experiments at 2.5 MPa of pressure of the Li2CuO2 solid state and ball milled samples under N2 and CO2 atmospheres. | ||
In a previous paper,19 it was already shown that Li2CuO2 is able to trap CO2 chemically at low temperatures in the presence of water vapor, where H2O works as a superficial catalytic intermediate, decreasing the activation energy of the CO2 chemisorption process. Therefore, the ball-milled Li2CuO2, possessing a larger surface area, is expected to present an interesting CO2 capture process in the Li2CuO2–CO2–H2O system at low temperatures (30 to 80 °C). Fig. 10 shows different isothermal experiments performed with the ball-milled Li2CuO2 sample. Isothermal experiments were performed at 40, 60 and 80 °C at different relative humidities (RH = 20%, 40%, 60%, and 80%). Independently of temperature, the weight gained increased as a function of RH with growing exponential trends. If these curves are compared to previously reported solid-state isotherms,19 the ball milled Li2CuO2 isotherms presented higher weight increments, except at 80 °C. Specifically at 80 °C and 80% RH, the Li2CuO2 solid-state and ball milled samples gained 37.6 and 31.8 wt%, respectively. Therefore, in order to further analyze the CO2 and H2O (hydroxides and water adsorbed) amounts, the isothermal products were analyzed and quantified by decomposition TG analysis. Fig. 11 shows the TG decomposition curves of the isothermal products treated at 80 °C with different RH, where different weight decrements are evidenced. In addition, as example, the derived thermogravimetric (DTG) curve of product treated at 80 °C and 20% RH was included. At low temperature, there is a slight, continuous weight decrement (around 1 to 3 wt%), which corresponds to the evaporation of adsorbed water. Then, between 340 and 440 °C, a dehydroxylation process is produced, as is evident in the DGT curve. In fact, this process was more marked at low RH, which suggests a lower CO2 capture, as the lithium atoms are responsible for these hydroxylated species. Finally, at T ≥ 600 °C, the main weight loss is produced by the decarbonation process. In the DTG curve, it can be seen that the decarbonation process occurred in two steps, between 580 and 690 °C and between 690 and 910 °C. This double process can be explained as the superficial and bulk decarbonation processes.
 | ||
| Fig. 10 Li2CuO2–CO2–H2O thermogravimetric isotherms performed with the Li2CuO2 ball milled sample at different temperatures (40, 60 and 80 °C) and RH (20%, 40%, 60% and 80%). | ||
 | ||
| Fig. 11 Decomposition TG curves of the Li2CuO2–CO2–H2O products treated isothermally at 80 °C and different RH (20% to 80%), and the DTG curve of the Li2CuO2 sample treated at 80 °C and 20% RH. | ||
Based on these quantitative results, the CO2 and H2O (hydroxides and water adsorbed) amounts were determined, as shown in Fig. 12. The adsorbed and chemisorbed H2O basically decrease with temperature. For example, at 80% RH, the amounts of water adsorbed and chemisorbed (as –OH species) were 4.2, 3.2 and 2.6 wt% at 40, 60 and 80 °C, respectively. This can be simply explained by the fact that at a higher temperature, H2O evaporation as well as the reactivity of the hydroxyl species increases, promoting the carbonation process. Conversely, the chemisorbed CO2 tended to increase as a function of temperature and RH, where the maximal amounts of CO2 captured were 6.1, 6.3 and 6.9 mmol g−1 at 40, 60 and 80 °C, respectively, in 80% RH. If these results are compared to previous reports,19 the CO2 amounts captured at 40 and 60 °C were higher in the present case (40 to 50%); however, the CO2 captured at 80 °C did not vary significantly. As in the previous case, the CO2 capture increments should be attributed to the ceramic hydroxylation process, which promotes the CO2 reactivity; the increased capture with the ball milled sample compared to the solid state sample is an effect of the surface area.
 | ||
| Fig. 12 Quantification of the CO2 and H2O (adsorbed water and surface hydroxylation) desorbed in the TG analyses. | ||
4. Conclusions
Li2CuO2 was microstructurally modified by a ball milling process, and different CO2 capture experiments were performed and compared to the Li2CuO2 solid-state sample. The XRD results evidenced that the Li2CuO2 crystalline structure was preserved after the ball milling process; however, the crystallite size, surface area and texture microstructure were modified. Later, the effects of these factors proved to be very important in the CO2 capture process under different physicochemical conditions. An initial dynamic CO2 capture experiment showed that these new microstructural features enhanced the CO2 capture in the following ways, in comparison to the Li2CuO2 solid state sample: (i) the CO2 chemisorption was significantly shifted to lower temperatures (T ≥ 80 °C) and (ii) the efficiency tended to increase.The Li2CuO2 microstructural changes were shown to have an important influence at low-moderate temperatures during the CO2 capture process; additionally, two different kinds of CO2 capture experiments were performed, with moderate pressure at moderate temperature or at low temperature in the presence of water vapor.
Moderate-pressure CO2 capture on Li2CuO2 showed that CO2 is chemisorbed, and much more CO2 is trapped than that chemisorbed at atmospheric pressure in the same temperature range. In fact, the highest chemisorption under these physicochemical conditions was achieved between 300 and 350 °C, where Li2CuO2 trapped 5.7 mmol of CO2 per gram of ceramic (63% of the maximum theoretical CO2 chemisorption capacity).
Simultaneously, the CO2 capture, in the presence of water vapor, was evaluated at low temperatures for the ball milled Li2CuO2 sample. As in the previous case, the microstructural modifications enhanced the CO2 chemisorption. Here, Li2CuO2 was able to chemisorb between 6.1 and 6.9 mmol of CO2 per gram of Li2CuO2 at temperatures between 40 and 80 °C. All these results strongly suggest that ball milled Li2CuO2 sample trapped more CO2 under these physicochemical conditions than the solid-state sample because of the microstructural modifications, where the crystallite size, surface area and solid–gas interphase changes enhanced the CO2 capture. Therefore, all these results strongly suggest that Li2CuO2 could be considered as a possible CO2 captor at low-moderate temperatures under different physicochemical conditions, as the CO2 amounts captured are comparable with other systems and materials. Moreover, in this temperature range, the CO2 capture is mostly produced by a physisorption process, while in the present case, CO2 is trapped chemically. Thus, this material may present different possible applications.
Acknowledgements
This work was financially supported by the projects SENER-CONACYT and PAPIIT-UNAM. H. A. Lara-García thanks CONACYT for their financial support. The authors thank A. Tejeda, D. Cabrero and J. Romero-Ibarra for their technical help.References
- S. D. Kenarsari, D. Yang, G. Jiang, S. Zhang, J. Wang, A. G. Russell, Q. Wei and M. Fan, RSC Adv., 2013, 3, 22739–22773 RSC.
- J. Wang, L. Huang, R. Yang, Z. Zhang, J. Wu, Y. Gao, Q. Wang, D. O'Hare and Z. Zhong, Energy Environ. Sci., 2014, 7, 3478–3518 CAS.
- X. Jiao, H. Li, L. Li, F. Xiao, N. Zhao and W. Wei, RSC Adv., 2014, 4, 47012–47020 RSC.
- L. Li, N. Zhao, W. Wei and Y. Sun, Fuel, 2013, 108, 112–130 CrossRef CAS.
- S. Choi, J. H. Drese and C. W. Jones, ChemSusChem, 2009, 2, 796–854 CrossRef CAS PubMed.
- X. Lu, D. Jin, S. Wei, Z. Wang, C. An and W. Guo, J. Mater. Chem. A, 2015, 3, 12118–12132 CAS.
- K. Nakagawa and T. Ohashi, J. Electrochem. Soc., 1998, 145, 1344–1346 CrossRef CAS.
- Q. Xiao, Y. Liu, Y. Zhong and W. Zhu, J. Mater. Chem., 2011, 21, 3838–3842 RSC.
- S. Wang, C. An and Q.-H. Zhang, J. Mater. Chem. A, 2013, 1, 3540–3550 CAS.
- T. Zhao, E. Ochoa-Fernández, M. Ronning and D. Chen, Chem. Mater., 2007, 19, 3294–3301 CrossRef CAS.
- Y. Duan, H. Pfeiffer, B. Li, I. C. Romero-Ibarra, D. C. Sorescu, D. R. Luebke and J. W. Halley, Phys. Chem. Chem. Phys., 2013, 15, 13538–13558 RSC.
- S. M. Amorim, M. D. Domenico, T. L. P. Dantas, H. J. José and R. F. P. M. Moreira, Chem. Eng. J., 2016, 283, 388–396 CrossRef CAS.
- M. Seggiani, M. Puccini and S. Vitolo, Int. J. Greenhouse Gas Control, 2011, 5, 741–748 CrossRef CAS.
- P. V. Subha, B. N. Nair, P. Hareesh, A. P. Mohamed, T. Yamaguchi, K. G. K. Warrier and U. S. Hareesh, J. Mater. Chem. A, 2014, 2, 12792–12798 CAS.
- M. Kato, K. Nakagawa, K. Essaki, Y. Maezawa, S. Takeda, R. Kogo and Y. Hagiwara, Int. J. Appl. Ceram. Technol., 2005, 2, 467–475 CrossRef CAS.
- F. Durán-Muñoz, I. C. Romero-Ibarra and H. Pfeiffer, J. Mater. Chem. A, 2013, 1, 3919–3925 Search PubMed.
- A. López-Ortiz, N. G. P. Rivera, A. R. Rojas and D. L. Gutierrez, Sep. Sci. Technol., 2005, 39, 3559–3572 CrossRef.
- P. Sánchez-Camacho, I. C. Romero-Ibarra, Y. Duan and H. Pfeiffer, J. Phys. Chem. C, 2014, 118, 19822–19832 Search PubMed.
- H. A. Lara-García, B. Alcántar-Vázquez, Y. Duan and H. Pfeiffer, RSC Adv., 2015, 5, 34157–34165 RSC.
- J. Fagerlund, J. Highfield and R. Zevenhoven, RSC Adv., 2012, 2, 10380–10393 RSC.
- L. Martinez-dlCruz and H. Pfeiffer, J. Phys. Chem. C, 2010, 114, 9453–9458 CAS.
- E. Ochoa-Fernández, T. Zhao, M. Rønning and D. Chen, J. Environ. Eng., 2009, 135, 397–403 CrossRef.
- R. Rodríguez-Mosqueda and H. Pfeiffer, J. Phys. Chem. C, 2013, 117, 13452–13461 Search PubMed.
- S. Zhang, Q. Zhang, H. Wang, Y. Ni and Z. Zhu, Int. J. Hydrogen Energy, 2014, 39, 17913–17920 CrossRef CAS.
- Q. Xiao, X. Tang, Y. Liu, Y. Zhong and W. Zhu, Chem. Eng. J., 2011, 174, 231–235 CrossRef CAS.
- J. Ortiz-Landeros, C. Gómez-Yáñez and H. Pfeiffer, J. Solid State Chem., 2011, 184, 2257–2262 CrossRef CAS.
- H. R. Radfarnia and M. C. Iliuta, Ind. Eng. Chem. Res., 2011, 50, 9295–9305 CrossRef CAS.
- H. R. Radfarnia and M. C. Iliuta, Sep. Purif. Technol., 2012, 93, 98–106 CrossRef CAS.
- M. Khokhani, R. B. Khomane and B. D. Kulkarni, J. Sol-Gel Sci. Technol., 2012, 61, 316–320 CrossRef CAS.
- I. C. Romero-Ibarra, J. Ortiz-Landeros and H. Pfeiffer, Thermochim. Acta, 2013, 567, 118–124 CrossRef CAS.
- A. Yang, H. Wang, W. Li and J. Shi, J. Am. Ceram. Soc., 2012, 95, 1818–1821 CrossRef CAS.
- P. R. Díaz-Herrera, M. J. Ramírez-Moreno and H. Pfeiffer, Chem. Eng. J., 2015, 264, 10–15 CrossRef.
- L. M. Palacios-Romero and H. Pfeiffer, Chem. Lett., 2008, 37, 862–863 CrossRef CAS.
- L. M. Palacios-Romero, E. Lima and H. Pfeiffer, J. Phys. Chem. A, 2009, 113, 193–198 CrossRef CAS PubMed.
- Y. Matsukura, T. Okumura, R. Kobayashi and K. Oh-ishi, Chem. Lett., 2010, 39, 966–967 CrossRef CAS.
- K. Oh-ishi, Y. Matsukura, T. Okumura, Y. Matsunaga and R. Kobayashi, J. Solid State Chem., 2014, 211, 162–169 CrossRef CAS.
- H. Kim, H. D. Jang and M. Choi, Chem. Eng. J., 2015, 280, 132–137 CrossRef CAS.
- S. Lowell, J. E. Shields and M. A. Thomas, Particle Technology Series, Kluwer Academic Publishers, London, 2004 Search PubMed.
- J. Rouquerol, F. Rouquerol and K. Sing, Adsorption by Powders & Porous Solids, Academic Press, London, 1999 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra06895b |
| This journal is © The Royal Society of Chemistry 2016 |


