Evolution of carbon impurities in solution-grown and sputtered Al:ZnO thin films exposed to UV light and damp heat degradation†
Peter Fuchs *,
Jérôme Steinhauser,
Enrico Avancini,
Yaroslav E. Romanyuk and
Ayodhya N. Tiwari
*,
Jérôme Steinhauser,
Enrico Avancini,
Yaroslav E. Romanyuk and
Ayodhya N. Tiwari
Empa, Swiss Federal Laboratories for Materials Science and Technology, Laboratory for Thin Films and Photovoltaics, Überlandstrasse 129, 8600 Dübendorf, Switzerland. E-mail: Peter.Fuchs@empa.ch; Fax: +41 58 765 40 42; Tel: +41 58 765 61 24
First published on 23rd May 2016
Abstract
The electrical properties of polycrystalline ZnO thin films with grain boundary limited charge transport depend significantly on the degree of chemisorbed adsorbate (CxOyHz) coverage at grain boundaries and on the surface. Here we report the evolution of carbon adsorbates in chemical bath deposited (CBD) aluminum doped ZnO (AZO) thin films during post-deposition UV light exposure and degradation in humid atmosphere. As CBD AZO intrinsically contains carbon impurities stemming from the addition of citrate during the deposition, sputtered AZO is examined in parallel as a nominally carbon-free reference. UV illumination decomposes citrate impurities in CBD AZO, but the residual carbon compounds are not effectively removed from the bulk part of the layer. At the same time, charge-trapping oxygen species at grain boundaries are desorbed, increasing the carrier density and mobility. Exposure to humid atmosphere decreases the electrical conductivity of CBD AZO, which is caused by the chemisorption of environmental oxygen species and the formation of zinc carbonate.
Introduction
Transparent conductive ZnO thin films can be processed from solutions by several deposition techniques (e.g. sol–gel based approaches,1 spray pyrolysis,2 chemical bath deposition3,4), however, their electrical performance and stability are significantly lower as compared to the established deposition methods (chemical vapor deposition, magnetron sputtering). To improve the conductivity of solution-processed layers, a high temperature post deposition treatment is typically applied which has the function to densify/sinter the layer, to remove residual organic impurities and/or to actively modify the surface/bulk composition by annealing in reducing atmosphere. A high temperature (T > ∼200 °C) post deposition treatment cannot be applied on temperature sensitive substrates such as polymer foils and solar cells. Therefore, alternative post deposition treatments such as UV illumination (photon energy > optical bandgap) have been developed to increase the conductivity.5–8 A UV treatment promotes the desorption of chemisorbed charged oxygen species, increasing the carrier density in the bulk and reducing potential barriers at grain boundaries.9–11 Furthermore, it could potentially lead to photolysis of ZnO,12 depending on the layer composition and treatment conditions.13 Carbon impurities have in this context been proposed to facilitate photolysis under UV illumination.14 UV light can also promote the decomposition of organic impurities, an effect which could lead to hydrogen doping of ZnO.5,15 The incomplete removal of residual organic impurities in the ZnO layer can be detrimental for the electrical performance stability, as carbon impurities may act as preferred adsorption sites for oxygen species (carbon oxidation).16,17 The formation of compounds of type (OH)x–Zn–(CO3)y was thereby proposed as a degradation mechanism,18 whereas the degradation kinetics depends on the temperature, film density and the oxygen species in the atmosphere amongst other factors.19–21 Although carbon impurities play an important role in both, the UV treatment and ZnO degradation, their exact nature has not been identified. In this work, we investigate chemical bath deposited (CBD) AZO layers that intrinsically contain carbon impurities because citrate is added as a structure directing agent during deposition. Thus CBD AZO films can serve as a model system to examine the role carbon impurities during the UV treatment and the degradation in humid atmosphere. Parallel investigations are conducted on RF-sputtered AZO as a nominally carbon-free reference.Experimental
CBD of ZnO
The deposition procedure for the non-intentionally-doped (n-i-d) CBD ZnO and CBD AZO baseline process are partly based on previously published procedures.22 Experimental details regarding the deposition can be found in the ESI.†AZO RF magnetron sputtering
RF magnetron sputtered AZO layers were deposited on soda lime glass (SLG) substrates without intentional substrate heating in a vacuum chamber with a direct facing target. Ceramic ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al2O3 (98
Al2O3 (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 wt%, purity 99.995%) targets with a diameter of 10.16 cm (4 in.) were sputtered in a mixed Ar/O2 atmosphere with a total pressure of 0.15
2 wt%, purity 99.995%) targets with a diameter of 10.16 cm (4 in.) were sputtered in a mixed Ar/O2 atmosphere with a total pressure of 0.15 ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pa and a power density of 2.5
Pa and a power density of 2.5 ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W cm−2.
W cm−2.
UV treatment and humidity exposure
To increase the electrical conductivity of the as-deposited films, a UV treatment was applied using a handheld UV lamp (Hoenle UV technology, UVAHAND 250 GS, 5 cm distance to substrate, 70 mW cm−2 UVA). The UV treatment was conducted for 10–15 min without intentional substrate heating. During the UV curing the substrate temperature did not exceed 150 °C at any time as measured by a thermocouple.To examine carbon compound formation in humid atmosphere, samples were exposed to a damp heat treatment at 80 °C/85% humidity in a SB1 300 WEISS climate chamber (further referred to as “damp heat”). Furthermore, samples were exposed to 100% relative humidity at room temperature in the dark by placing them in a closed box together with cups filled with deionized water.
Characterization
The sheet resistance of the ZnO thin films was measured by a four-probe method with a Nagy meter SD-600. For sheet resistances above 200 kΩ sq−1, the resistance of the layer was estimated with a multimeter, leading to approximate values as no sample geometry correction was applied. Hall measurements were conducted with an Ecopia HMS 3000 setup at room temperature in the dark using a van der Pauw contact geometry. UV/Vis measurements were performed with a Shimadzu UV-3600 spectrophotometer with an integrating sphere. Transmittance values were measured for the film-glass substrate stack against air reference.Fourier transform infrared spectroscopy (FTIR) measurements were conducted with a BRUKER single reflection attenuated total reflection (ATR) unit (diamond ATR crystal) incorporated in a BRUKER Tensor 27 spectrometer. A diamond crystal attenuated total reflection setup was used to enable direct investigation of the ZnO films on the non-IR-transparent glass substrates. The samples were mechanically pressed on the diamond crystal and probed with unpolarized light at an angle of incidence of 45°. The spectrometer was purged with CO2/H2O-free air.
X-ray Photoelectron Spectroscopy (XPS) measurements were performed using a Quantum2000 from Physical Electronics with a monochromatic Al Kα source (1486.6 eV). The work function of the instrument was calibrated on a regular basis to a binding energy of 83.95 eV (FWHM = 0.8 eV) for the Au 4f5/2 peak and the linearity of the energy scale is checked according to ISO 15472. The presented spectra were recorded with an energy step size of 0.2 eV and a pass energy of 46.95 eV (FWHM of Au 4f5/2 = 1.07 eV under the given conditions). An electron flood gun operated at 2.5 eV and an ion neutralizer using Ar+ of approx. 1 eV were used to minimize the fluctuations of the binding energy values due to sample charging. Spectra were recorded at a base pressure below 8 × 10−9 mbar. A surface sputter depth profile was conducted by Ar+ sputtering at 0.5 keV for 60 s, which leads to an estimated material removal in the order of ∼2 nm min−1. If not otherwise mentioned, detailed spectra presented in this work have been acquired without any pre-acquisition sputtering or prolonged (>5 min) pre-acquisition X-ray beam exposure.
Secondary Ion Mass Spectroscopy (SIMS) depth profiling data were obtained with a time-of-flight (TOF) SIMS 5 system from ION-TOF. Bi ions were used as primary ions and negative ions were detected. Sputtering was performed using Cs+ sputtering ions with 1 keV ion energy, 50 nA ion current and a 300 × 300 μm2 raster size. An area of 100 × 100 μm2 was analysed using Bi3+ ions with 25 keV ion energy.
Results and discussion
Electrical properties
As deposited CBD ZnO samples exhibit electron mobilities μ < ≈ 1 cm2 V−1 s−1 and carrier densities in the order of n ≈ 1 × 1018 cm−3. The conductivity of as deposited CBD ZnO samples increases by a factor ∼103 upon UV treatment, caused by a combined increase in carrier density and mobility (Table 1). N-i-d ZnO layers have a higher mobility than AZO, which can be attributed to reduced grain boundary scattering due to the increased film thickness for the n-i-d baseline (extending columnar growth leads to a reduced grain boundary density, see Fig. 1a and b). Limitation of electronic transport through grain boundary scattering could also be confirmed by growing even thicker layers (9 μm thick n-i-d) where mobilities up to μ = 19.5 cm2 V−1 s−1 were measured.| d [μm] | As deposited | UV treated | |||
|---|---|---|---|---|---|
| ρ [Ωcm] | ρ [10−3 Ω cm] | n [1020 cm−3] | μ [cm2 V−1 s−1] | ||
| a Average values and standard deviations are calculated for each parameter from twelve nominally identical deposition runs (d = film thickness, ρ = resistivity, n = carrier density, μ = mobility). | |||||
| CBD AZO | 1.4 ± 0.1 | 4.1 ± 2.5 | 4.1 ± 0.5 | 1.7 ± 0.2 | 9.1 ± 0.8 |
| CBD n-i-d ZnO | 3.2 ± 0.2 | 8.5 ± 4.7 | 5.1 ± 0.5 | 1.1 ± 0.1 | 11.3 ± 1.1 |
| Sputtered AZO | 0.6 | 0.8 × 10−3 | 0.8 | 3.6 | 21.6 |
UV treated samples are unstable when exposed to atmospheric oxygen species. Fig. 1d gives an overview on the resistance increase of UV treated samples exposed to different atmospheres/temperatures. Samples exposed to damp heat degrade very fast (factor of ∼104 resistivity increase within hours). The onset of degradation is characterized by an immediate drop of the mobility below the measurement limit. The decrease in carrier density can be observed by the reduction of free carrier absorption in the near infrared (Fig. 1e, ESI Fig. S1†). Samples exposed to 100% humidity at room temperature in the dark also degrade quickly. Encapsulated samples (epoxy encapsulation) or samples stored in reduced atmosphere ∼1 mbar degrade much slower, as predicted by the oxygen species adsorption model.9,10
Evolution of carbon impurities during UV treatment and damp heat exposure
As deposited, UV treated and damp heat treated samples were examined with FTIR spectroscopy to characterize carbon compounds in the bulk part of the ZnO layer. Assuming a refractive index of 1.9 for ZnO (assumption is valid for ZnO with a low carrier density in the wavenumber range 4000–2500 cm−1),23 the total internal reflection condition at the ZnO–diamond interface is not matched for the given FTIR-ATR setup. Therefore, the whole film is probed for as deposited CBD ZnO layers while total internal reflection takes place at the ZnO–glass interface. Characteristic peaks for citrate occur in the wavenumber range 1200–1700 cm−1 (magnified region Fig. 2a). The main peaks correspond to two asymmetric (νas ∼ 1565/1620 cm−1) and symmetric (νs ∼ 1390/1425 cm−1) carboxylate R–COO− stretching vibrations. The double peaks indicate at least two coordination states where the higher wavenumber components (1620/1425 cm−1) are attributed to a monodentate inner sphere complex.24 The lower wavenumber compound (1565/1390 cm−1) might reflect a bridging coordination state, showing a slightly lower peak separation (νas − νs).25,26 Complete IR penetration into the layer is manifested by peaks at 760/905 cm−1 which are assigned to the SLG glass substrate. The small peak at ∼690 cm−1 comes from an Al(O–OH) compound as it is not found on n-i-d doped layers. Table S1 in the ESI† section gives an overview on the attribution of all detected peaks to the respective vibration modes and comparison to literature values.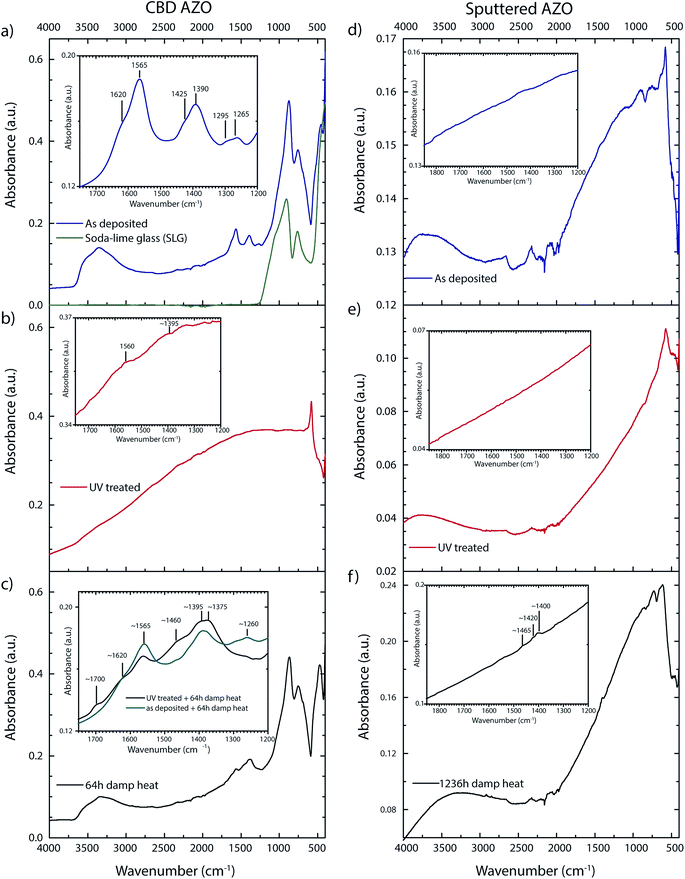 | ||
| Fig. 2 FTIR spectra of CBD AZO: (a) as deposited, (b) UV treated, (c) after damp heat. FTIR spectra of sputtered AZO: (d) as deposited, (e) UV treated, (f) after damp heat. | ||
For UV treated samples the IR beam does not probe the whole layer anymore because the increased free carrier density leads to a shift of the reflection edge to the NIR (the complex refractive index depends amongst the carrier density on additional factors such as the volume fraction of depleted material27). The incoming beam is to a significant degree reflected at the ZnO–diamond interface, while the transmitted part is nearly fully adsorbed. Any carbon impurities lie around the detection limit (Fig. 2b). It will be demonstrated later that carbon compounds are still present even though not detected by IR. The only clear peak at ∼580 cm−1 is attributed to ZnO vibration modes.28 Interestingly, this peak appears only after the UV treatment.
Samples exposed to the damp heat treatment show a significant decrease in the carrier density, which goes along with an increase in the IR transparency, thus carbon compounds can again be measured throughout the layer (Fig. 2c). Carboxyl impurities are found to a significant degree, though changes in intensity ratios and new peaks indicate either a decomposition of citrate or the adsorption of carboxyl species during the damp heat treatment. Both, a peak at ∼1700 cm−1 and a broad peak around 1460 cm−1 only appear in layers which have undergone a UV treatment before the damp heat treatment (Fig. 2c inset). The peak at 1700 cm−1 is attributed to free carboxylic acid group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), indicating citrate decomposition, which is in accordance with a report on thermally decomposed zinc ammonium citrate.29 Citrate decomposition is enabled by the presence of ZnO as pure citrate compounds (e.g. sodium citrate tribasic) did not decompose under the same UV treatment (see Fig. S2 (ESI†)). The broad peak at 1460 cm−1 could be assigned either to C–H vibration modes, ν(C–O) of zinc acetate hydrate30 or zinc carbonate31 which would also account for the broadening of the shoulder at ∼1390 cm−1. UV treated samples which were exposed to 100% humidity at room temperature in the dark showed exactly the same vibration pattern as UV-damp heat treated samples.
O), indicating citrate decomposition, which is in accordance with a report on thermally decomposed zinc ammonium citrate.29 Citrate decomposition is enabled by the presence of ZnO as pure citrate compounds (e.g. sodium citrate tribasic) did not decompose under the same UV treatment (see Fig. S2 (ESI†)). The broad peak at 1460 cm−1 could be assigned either to C–H vibration modes, ν(C–O) of zinc acetate hydrate30 or zinc carbonate31 which would also account for the broadening of the shoulder at ∼1390 cm−1. UV treated samples which were exposed to 100% humidity at room temperature in the dark showed exactly the same vibration pattern as UV-damp heat treated samples.
Negative ion SIMS depth profiles of n-i-d CBD ZnO samples were acquired to confirm the incomplete removal of carbon compounds during the UV treatment. A close to constant carbon concentration is detected in the as deposited layer (Fig. 3a). After the UV treatment a slight decrease in the carbon concentration towards the surface is found (Fig. 3b), which agrees well with the decreased carbon concentration observed in XPS measurements which will be discussed later. Carbon compounds are not effectively removed in the bulk part of the layer, presumably due to the combined effect of limited UV penetration depth and limited out-diffusion of carbon species. This is not in agreement with an observation made by Wagata et al. for a similar system (spin spray deposited ZnO layers), which can probably be attributed to different film densities or to the fact that limited IR penetration for highly doped layers was not taken into account in that work when conducting the FTIR measurements.5
 | ||
| Fig. 3 Negative ion SIMS depth profile through ∼2 μm thick CBD n-i-d ZnO layers. (a) As deposited (b) UV treated. | ||
For sputtered AZO, not the whole layer is probed by the IR beam due to the high carrier density (n ∼ 3.6 × 1020 cm−3). Carbon impurities lie below the detection limit, the variations between as deposited (Fig. 2d) and UV treated (Fig. 2e) are caused by slight distance variations in ATR crystal-sample contact. The peaks in-between 2000–2500 cm−1 are a diamond crystal measurement artefact. Carbon species related peaks are detected only after prolonged (>1000 h, n ∼ 3.3 × 1020 cm−3) damp heat exposure (Fig. 2f). However, these peaks are partially attributed to hydrocarbon impurities originating from the damp heat chamber environment (C–H vibration modes at 2920 cm−1, 2850 cm−1, 1465 cm−1). The formation of zinc carbonate basic (Zn5(CO3)2(OH)6), which was suggested as a degradation product in an earlier work,18 could not be detected for damp heat treated AZO (zinc carbonate basic would show a dominant peak at 1505 cm−1,31,32 for a reference spectra see ESI Fig. S2†). The presence of zinc carbonate (ZnCO3)31 is not excluded, but also not unambiguously confirmed, due to overlap with C–H vibration modes.
Surface sensitive information on the type of carbon–oxygen adsorbates can be acquired by XPS measurements. The O1s peak reflects qualitatively very well the removal of adsorbates upon UV treatment and the increased adsorption of oxygen species during exposure to humid atmosphere (Fig. 4). However, the O1s peak is not analyzed in detail due to peak overlap of several Zn–C–O compounds (e.g. Zn–O (bulk): 530.5 eV,33,34 Zn–OH: 532.0 eV,34 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O: 532.0 eV,35 C–OH: 532.6 eV (ref. 35)) which make a reliable peak fitting very difficult.
O: 532.0 eV,35 C–OH: 532.6 eV (ref. 35)) which make a reliable peak fitting very difficult.
A better estimate of the adsorbed Zn–Cx–Oy–Hz compounds can be gained from the C1s peak. Fig. 5 gives an overview on the carbon surface species detected at different sample treating stages. As the adsorbed carbon species are sensitive to X-ray irradiation (beam damage), detailed spectra were acquired before the acquisition of a survey (“as inserted”) followed by an additional detailed spectra run to estimate beam damage after the survey (∼1 h X-ray exposure). For spectrum comparison, the hydrocarbon component peaks (CxHy) species are aligned to 284.8 eV. The uncorrected CxHy position depends on the sample treatment stage and lies in the range 285.4–286.1 eV without pre-acquisition sputtering (see therefore B. V. Crist).37 All peaks shift towards lower binding energy numbers with sputtering/illumination time, which is correlating with the removal of the adsorbate layer (irradiation for ∼1 h shifts the peaks ∼0.3 eV, Ar+ sputtering for 60 s shifts ∼1 eV). Measures for charge compensation did not significantly alter peak positions as compared to uncompensated samples (shifts below 0.2 eV).
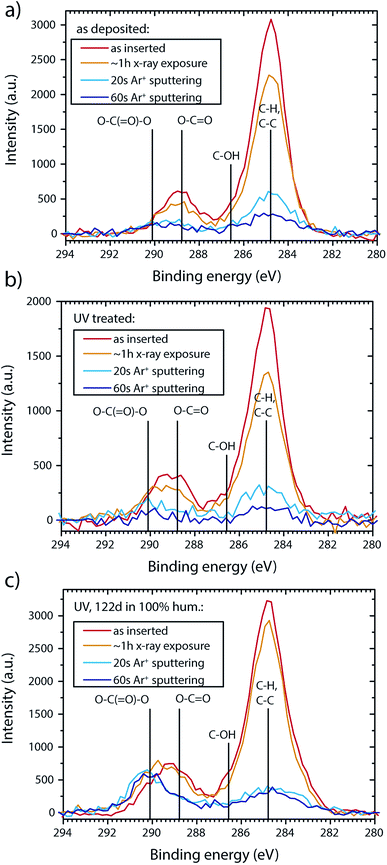 | ||
Fig. 5 XPS C1s sputter depth profile for (a) as deposited CBD AZO. (b) UV treated CBD AZO (c) CBD AZO exposed to 100% humidity at room temperature in the dark for 122 d. Lines indicate literature peak position as a guide for the eye: CxHy: 284.8 eV (peak alignment) C–OH: 286.3 eV,35 carboxyl (O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O): 288.8 eV,35 carbonate (O–C( O): 288.8 eV,35 carbonate (O–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–O): 290.1 eV.36 O)–O): 290.1 eV.36 | ||
The C1s of as deposited samples features the expected peaks for adventitious carbon and citrate (C–H, C–OH, COO(H, Zn), Fig. 5a). While a strong decrease in the CxHy carbon level is observed for UV treated samples, surface carbon removal is incomplete (Fig. 5b). Exposure to 100% relative humidity at room temperature leads to carbonate formation (carbonate peak at ∼290.1 eV,35,36 Fig. 5c). The carbonate peak is clearly apparent after Ar sputtering (20 s at 0.5 keV), which removes most of the adventitious surface carbon compounds.
Fig. 6 presents carbon compound formation on samples that were exposed to humidity at elevated temperature (damp heat). Thereby, a carbonate peak is not observed for sputtered AZO as well as CBD AZO (Fig. 6a). The only change in chemical state is found for the carboxyl (COO(H, Zn−)) adsorbate peak of CBD AZO, which shifts from 289.0 eV (as deposited) to lower binding energies with increasing damp heat treatment time (Fig. 6b). This shift correlates with the surface sodium content, possibly indicating the formation of a sodium carboxylate compound (the basis for this claim is a reference spectrum of sodium citrate, where the COO–Na peak is found at 288.2 eV).25 While sodium carboxylate formation is caused by the particular choice of substrate (Na out-diffusion from the soda lime glass at elevated temperature) in this study, it outlines the much faster ion diffusion in CBD AZO when compared to sputtered AZO, where the sodium surface concentration remained below the detection limit for up to at least 588 h damp heat exposure.
A further measure for the direct chemical environment of Zn is the modified Auger parameter (α = binding energy (Zn2p3/2) + kinetic energy of highest intensity ZnLMM line),38 where tabulated values for Zn–Cx–Oy–Hz species are available (α(ZnO) = 2010.1–2010.3 eV, α(ZnCO3) = 2009.7–2009.8 eV, α(Zn5(OH)6(CO3)2 = 2009.4–2009.7 eV and Zn(OH)2 = 2009.2 eV)).34,36 To determine the kinetic energy of highest intensity ZnLMM line, a Shirley background correction was applied to the Zn L3M4,5M4,5 peak (Fig. 7). Then the peak was deconvoluted into two Gaussian–Lorentzian peaks (fit restriction: 3.2 eV for peak separation,39,40 the peak fine structure is neglected41). The high binding energy component is selected for the modified Auger parameter calculation. This simple fit is less accurate for highly doped layers (fit example in ESI Fig. S3†), due to an additional shoulder contribution on the lower BE side of the most intense Auger component, presumably caused by free electron screening effects,42 which leads to a slight overestimation of the auger parameter with the given fit. Table 2 presents the calculated modified Auger parameter. For as deposited and UV treated sputtered AZO the calculated α lies at 2010.4 eV, which is in agreement or slightly above literature values.36,40,43 After Ar sputtering for 60 s/0.5 keV, an Auger parameter of 2010.2 eV was measured independent of the processing history. For as deposited CBD AZO α lies significantly lower at 2009.5 eV, indicating a Zn–carboxyl–hydroxyl compound. The UV treatment increases α, probably caused by the increased free carrier concentration, yet the value of sputtered ZnO is not reached. For damp heat treated CBD samples the Auger parameter is not calculated here as there is a sodium Auger contribution at ∼497.5 eV which disables a reliable peak deconvolution. Qualitatively a shift towards zinc carboxylate compounds is still apparent (Fig. 7).
| Auger parameter | As deposited [eV] | UV treated [eV] | Damp heat [eV] |
|---|---|---|---|
| a Standard deviations are calculated from measurements on three nominally identically processed/fitted samples, they do not represent absolute measurements errors. Ar+ sputtering was conducted at 0.5 keV for 60 s. | |||
| CBD AZO | 2009.5 ± 0.1 | 2009.7 ± 0.2 | — (see text) |
| CBD AZO 60 s Ar+ sput. | 2009.7 ± 0.2 | 2009.8 ± 0.2 | — (see text) |
| Sput. AZO | 2010.4 ± 0.1 | 2010.4 ± 0.1 | 2009.9 ± 0.1 |
| Sput. AZO 60 s Ar+ sput. | 2010.2 ± 0.1 | 2010.2 ± 0.1 | 2010.2 ± 0.1 |
Conclusions
A significant amount of carbon impurities stemming from the addition of citrate as a structure directing agent is detected in as deposited CBD AZO. The post deposition UV treatment leads to the decomposition of citrate, yet bulk carbon residuals are not effectively removed, presumably due to limited UV penetration depth and out-diffusion. At the same time, UV illumination desorbs charged oxygen species at grain boundaries, increasing the carrier density and mobility, leaving the film non transparent to IR and thus preventing direct investigation of decomposition products by means of FTIR. Considering the compounds found in samples degraded in humid atmosphere, free carboxylic acids are likely a citrate decomposition product. The effect of UV treatment on sputtered AZO is negligible due to the higher carrier density, denser microstructure and lower level of carbon impurities in the as deposited state.CBD AZO degrades much faster than sputtered AZO which can be explained by the less dense columnar structure of CBD AZO and the overall lower carrier density which increases the contribution of grain boundary scattering. A degradation acceleration effect due to carbon impurities cannot be excluded. Surface carbonate formation was observed for CBD AZO samples exposed to 100% humidity at room temperature but not for damp heat treated samples. Sputtered AZO degradation is accompanied by the adsorption of atmospheric carbon but the expected final decomposition product – zinc carbonate basic – could not unambiguously be detected after the applied damp heat tests.
Acknowledgements
Financial support from the Swiss Federal Office of Energy (BFE, Project “NOVAZOLAR”) and the Competence Center Energy and Mobility (CCEM, Project “CONNECT-PV”) is gratefully acknowledged.References
- H. Damm, P. Adriaensens, C. De Dobbelaere, B. Capon, K. Elen, J. Drijkoningen, B. Conings, J. V. Manca, J. D. Haen, C. Detavernier, P. C. M. M. Magusin, J. Hadermann, A. Hardy and M. K. Van Bael, Chem. Mater., 2014, 26, 5839–5851 CrossRef CAS.
- J. Aranovich, J. Vac. Sci. Technol., 1979, 16, 994 CrossRef CAS.
- M. Miyake, H. Fukui and T. Hirato, Phys. Status Solidi, 2012, 209, 945–948 CrossRef CAS.
- M. Kevin and G. W. Ho, J. Mater. Chem. A, 2013, 1, 14239 CAS.
- H. Wagata, N. Ohashi, K. Katsumata, H. Segawa, Y. Wada, H. Yoshikawa, S. Ueda, K. Okada and N. Matsushita, J. Mater. Chem., 2012, 22, 20706 RSC.
- L. Ding, S. Nicolay, J. Steinhauser, U. Kroll and C. Ballif, Adv. Funct. Mater., 2013, 23, 5177–5182 CrossRef CAS.
- H. Hagendorfer, K. Lienau, S. Nishiwaki, C. M. Fella, L. Kranz, A. R. Uhl, D. Jaeger, L. Luo, C. Gretener, S. Buecheler, Y. E. Romanyuk and A. N. Tiwari, Adv. Mater., 2013, 632–636 Search PubMed.
- A. T. Vai, V. L. Kuznetsov, J. R. Dilworth and P. P. Edwards, J. Mater. Chem. C, 2014, 2, 9643–9652 RSC.
- S. R. Morrison, Adv. Catal., 1955, 7, 259–301 CAS.
- G. Heiland, E. Mollwo and F. Stöckmann, Solid State Phys., 1959, 8, 191–323 CAS.
- T. L. Tansley and D. F. Neely, Thin Solid Films, 1984, 121, 95–107 CrossRef CAS.
- R. J. Collins and D. G. Thomas, Phys. Rev., 1958, 112, 388–395 CrossRef CAS.
- R. I. Bickley, G. Heiland, W. Hirschwald, E. Thull, F. Steinbach, R. Harborth, W. Bauer, A. Hausmann, A. J. Tench, J. Cunningham, M. W. Roberts, T. B. Grimley, D. Menzel and H. P. Boehm, Faraday Discuss. Chem. Soc., 1974, 58, 175–184 RSC.
- J. Bao, I. Shalish, Z. Su, R. Gurwitz, F. Capasso, X. Wang and Z. Ren, Nanoscale Res. Lett., 2011, 6, 404 CrossRef PubMed.
- H. Wagata, N. Ohashi, T. Taniguchi, K. I. Katsumata, K. Okada and N. Matsushita, Cryst. Growth Des., 2010, 10, 4968–4975 CAS.
- Y. Shapira, S. M. Cox and D. Lichtman, Surf. Sci., 1976, 54, 43–59 CrossRef CAS.
- J. Lagowski, E. S. Sproles and H. C. Gatos, J. Appl. Phys., 1977, 48, 3566 CrossRef CAS.
- M. Theelen, T. Boumans, F. Stegeman, F. Colberts, A. Illiberi, J. Van Berkum, N. Barreau, Z. Vroon and M. Zeman, Thin Solid Films, 2014, 550, 530–540 CrossRef CAS.
- T. Tohsophon, J. Hüpkes, S. Calnan, W. Reetz, B. Rech, W. Beyer and N. Sirikulrat, Thin Solid Films, 2006, 511–512, 673–677 CrossRef CAS.
- D. Greiner, S. E. Gledhill, C. Köble, J. Krammer and R. Klenk, Thin Solid Films, 2011, 520, 1285–1290 CrossRef CAS.
- J. Hüpkes, J. I. Owen, M. Wimmer, F. Ruske, D. Greiner, R. Klenk, U. Zastrow and J. Hotovy, Thin Solid Films, 2014, 555, 48–52 CrossRef.
- P. Fuchs, H. Hagendorfer, Y. E. Romanyuk and A. N. Tiwari, Phys. Status Solidi, 2015, 212, 51–55 CrossRef CAS.
- W. L. Bond, J. Appl. Phys., 1965, 36, 1674–1677 CrossRef CAS.
- N. J. Nicholas, Control of ZnO Crystal Morphology through Face Specific Adsorption, Ph.D. Dissertation, University of Melbourne, Australia, 2011.
- J. W. Park and J. S. Shumaker-Parry, J. Am. Chem. Soc., 2014, 136, 1907–1921 CrossRef CAS PubMed.
- G. Deacon, Coord. Chem. Rev., 1980, 33, 227–250 CrossRef CAS.
- P. Prunici, F. U. Hamelmann, W. Beyer, H. Kurz and H. Stiebig, J. Appl. Phys., 2013, 113, 123104 CrossRef.
- H. Morkoç and Ü. Özgür, in Zinc Oxide, Wiley-VCH Verlag GmbH & Co. KGaA, 2009, pp. 1–76 Search PubMed.
- K. V. Van Werde, D. Mondelaers, G. Vanhoyland, D. Nelis and M. K. Van Bael, J. Mater. Sci., 2002, 7, 81–88 CrossRef.
- V. Koleva and D. Stoilova, J. Mol. Struct., 2002, 611, 1–8 CrossRef CAS.
- M. C. Hales and R. L. Frost, Polyhedron, 2007, 26, 4955–4962 CrossRef CAS.
- D. Stoilova, V. Koleva and V. Vassileva, Spectrochim. Acta, Part A, 2002, 58, 2051–2059 CrossRef CAS.
- W. F. Stickle, P. E. Sobol and K. D. Bomben, Handbook of X-ray Photoelectron Spectroscopy, Perkin-Elmer Corporation, Minnesota, 1992 Search PubMed.
- G. Deroubaix and P. Marcus, Surf. Interface Anal., 1992, 18, 39–46 CrossRef CAS.
- B. D. Ratner and D. G. Castner, Electron spectroscopy for chemical analysis, John Wiley & Sons, Ltd., 2009 Search PubMed.
- L. S. Dake, D. R. Baer and J. M. Zachara, Surf. Interface Anal., 1989, 14, 71–75 CrossRef CAS.
- B. V. Crist, J. AES XPS Reports, 2007, 1, 9–10.
- C. D. Wagner, J. Electron Spectrosc. Relat. Phenom., 1988, 47, 283–313 CrossRef CAS.
- G. Schon, J. Electron Spectrosc. Relat. Phenom., 1973, 2, 75–86 CrossRef CAS.
- B. R. Strohmeier and D. M. Hercules, J. Catal., 1984, 86, 266–279 CrossRef CAS.
- P. Weightman, J. F. McGilp and C. E. Johnson, J. Phys. C: Solid State Phys., 1976, 9, L585 CrossRef CAS.
- A. Klein and F. Sauberlich, in Transparent Conductive Zinc Oxide, Springer, 2008, pp. 125–185 Search PubMed.
- T. Adler, M. Botros, W. Witte, D. Hariskos, R. Menner, M. Powalla and A. Klein, Phys. Status Solidi, 2014, 9, 1972–1980 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details with regard to the chemical bath deposition of ZnO, UV-Vis data of CBD AZO, FTIR literature reference data for the ZnO-citrate system, ATR-IR spectra of citrate reference compounds and XPS ZnLMM peak fitting procedure. See DOI: 10.1039/c6ra06861h |
| This journal is © The Royal Society of Chemistry 2016 |




