High energy density of quasi-solid-state supercapacitor based on redox-mediated gel polymer electrolyte
Kanjun Sun†
*ab,
Feitian Ran†b,
Guohu Zhaoa,
Yanrong Zhua,
Yanping Zhenga,
Mingguang Maa,
Xiaoping Zhenga,
Guofu Ma*b and
Ziqiang Leib
aCollege of Chemistry and Environmental Science, Lanzhou City University, Lanzhou 730070, China. E-mail: sunkj@lzcn.edu.cn
bKey Laboratory of Eco-Environment-Related Polymer Materials of Ministry of Education, Key Laboratory of Polymer Materials of Gansu Province, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou 730070, China. E-mail: magf@nwnu.edu.cn
First published on 2nd June 2016
Abstract
A novel redox-mediated gel polymer (PVA–H2SO4–ARS) is prepared by introducing alizarin red S (ARS) into a polyvinyl alcohol–sulphuric acid (PVA–H2SO4) gel polymer system, and a symmetric supercapacitor using the gel polymer as electrolyte and activated carbon as electrode is also assembled. The PVA–H2SO4–ARS gel polymer has excellent bending, compressing and stretching mechanical properties. The introduction of ARS increases the ionic conductivity of the gel polymer, and improves the pseudocapacitance of the supercapacitor. As expected, the PVA–H2SO4–ARS gel polymer electrolyte has a high conductivity of 33.3 mS cm−1, and the supercapacitor with PVA–H2SO4–ARS electrolyte exhibits a larger electrode specific capacitance (441 F g−1) than the one with PVA–H2SO4 electrolyte (160 F g−1) at the same current density of 0.5 A g−1. Simultaneously, the supercapacitor with PVA–H2SO4–ARS electrolyte exhibits high energy density (39.4 W h kg−1) and good charge–discharge stability. Therefore, this novel electrolyte has good prospects for improving the electrochemical performance of an energy storage device.
1. Introduction
With the tremendously increasing power and energy demands for miniaturized and flexible electronic devices such as deformable displays, self-powered sensor networks, and wearable devices, the design and fabrication of compatible energy storage devices have become new challenges.1–4 Among the various energy storage technologies, supercapacitors (SCs) have recently become promising candidates in the energy storage areas of charge storage devices and are used for various potential applications owing to their higher power densities, higher charge–discharge rates, stable cycling performance and safer operation than batteries.5,6 SCs are already being used in different applications such as in digital communication systems, portable electronic devices and medical applications, memory backup systems, and hybrid electric vehicles.7The performances of supercapacitors highly depend on the electrode materials.8 In spite of this, the electrolyte is also a critical factor that can significantly affect the performance of SCs even highly flexible devices.9 However, their wide applications are commonly diminished by certain problems such as electrolyte leakage, corrosion, and packing since commercially available SCs are fabricated by using liquid electrolytes. Moreover, the liquid electrolytes release some hazardous byproduct into the environment.5 Therefore, the special configuration design of thin and flexible layer of gel polymer electrolyte is considered to be one of the most effective strategy because of the intrinsic properties of polymer electrolyte, such as thin-film forming ability, flexibility as well as the relatively high ionic conductivity and wide electrochemical window.10 Besides, the supercapacitors integrating electrodes, solid electrolyte and separator into a solid whole, have many obvious advantages such as environmental friendliness, portability, flexibility, and stability.11
At present, various polymer matrices, such as anionic polyurethane acrylates/polyacrylamide (aPUA/PAAM),12 polymethylmethacrylate (PMMA),13 polyvinylidene fluoride (PVDF)14 and polyvinyl alcohol (PVA)15 etc. have been widely developed as candidate materials for the preparation of gel polymer electrolytes. There is a remarkable ions migration efficiency and mechanical robustness in these gel polymer electrolytes, which provides breakthrough electrochemical performances. Especially, PVA have been developed as the main substrate for applications in SCs.16 It is well known that acidic, alkali and neutral solutions are mostly used as electrolyte due to their high ionic conductivity and low price, but incorporation them into the polymer matrices cannot improve the specific capacitance and energy density of SCs due to their low operating potential (the water decomposition potential of 1.23 V).15
Recently, some studies have proven that the incorporation of redox-active molecules into the electrolyte has efficiently enhanced the capacitive performance of SCs, and this type of electrolyte is considered as the redox additive or mediated electrolyte. These redox additives or compounds are directly involved in the electron transfer redox reaction, and the performance of the SCs are improved by their surface pseudocapacitive contribution at the electrode–electrolyte interface.17 For examples, Yu et al. have prepared the redox additive of PVA–KOH polymer electrolyte using KI, the PVA–KOH–KI polymer electrolyte showed improved capacitance and energy density of 236.9 F g−1 and 15.34 W h kg−1, which are nearly 74.28% more than for PVA–KOH electrolyte.18 The same authors have used p-benzenediol as redox mediator in PVA–H2SO4 gel electrolyte, the large specific capacitance (474.29 F g−1) and high energy density (11.31 W h kg−1) was obtained.19 Yu et al. have incorporated redox mediator MB into polyvinyl alcohol/polyvinyl pyrrolidone (PVA/PVP) blend host to prepared a gel polymer electrolyte (PVA–PVP–H2SO4–MB) for supercapacitors, the supercapacitor shows a high electrode specific capacitance of 328 F g−1 and high energy density of 10.3 W h kg−1.20 The excellent performance of these electrolyte inspire us to search for more efficient redox mediator to prepare the gel polymer electrolytes for high-performance supercapacitors.
Alizarin red S (ARS) is an anthraquinone dye used in textile industry since early antiquity because it contains hydroxyls groups that make it soluble in water and with great affinity for wool and silk without the aid of auxiliary binding agents,21 and recently it has been widely used as a redox pH sensor component, for biological staining, for drug analysis, and in sensing.22 To the best of our knowledge, practical applications of ARS as an redox mediator in electrolyte material for supercapacitor configurations have not been reported.
Herein, we developed a novel PVA–H2SO4–ARS gel polymer electrolyte with high conductivity and considerable mechanical properties by solution freezedrying method, and it was assembled with activated carbon electrodes to form a supercapacitor. The electrochemical properties and pseudocapacitive effect for the supercapacitor were investigated by cyclic voltammetry, galvanostatic charge–discharge, and electrochemical impedance spectroscopy techniques.
2. Experimental
2.1. Materials
Polyvinyl alcohol (PVA, Aladdin Co., China, molecular weight 44.05 MW, alcoholysis: 99.8–100%), sulphuric acid (H2SO4, Beijing north fine chemicals co., China), alizarin red S (ARS, Shanghai Zhongtai chemical reagent co., China), and activated carbon (AC, Shanghai Sino Tech Investment Management Co., China, specific surface area of 2000 ± m2 g−1, pore size of 2.0–2.2 nm) were purchased and used without further purification, unless otherwise specified. All other chemical reagents were of analytical grade.2.2. Preparation of gel polymer electrolyte
As our previous report,23 gel polymer electrolyte can be formed using PVA by a conventional solution/mixing-casting method. The PVA/H2SO4 gel polymer electrolyte and redox mediated PVA/H2SO4–ARS gel polymer electrolyte were prepared as follows. Firstly, PVA (1.0 g) was dissolved in 10 mL distilled water with constant agitation at 85 °C for 2 h to form a homogeneous and clear solution. Then, 10 mL of aqueous solution containing H2SO4 (2.0 g) and ARS (0–0.90 g) was added to the above solution under constant stirring, and it was kept stirring until the formation of a orange gel-like solution was observed. Subsequently, the resultant mixture was poured into a to 90 mm plastic Petri dish, and the dish was frozen at −25 °C for 12 h and thawed at room temperature for 12 h. The freeze-thaw cycles were repeated 2 times to obtain the PVA–H2SO4–ARS gel polymer. By the freeze-thaw method, the polymer chains of PVA can come into contact with each other, begin to overlap, and entangle reciprocally with the sublimation of ice, which leads to the formation of a stable gel polymer.16 The PVA–H2SO4 gel polymer without ARS was also prepared using the same method for comparison.2.3. Preparation of activated carbon electrode
Initially, the slurry was prepared by mixing of AC (16 mg, 80%), acetylene black (2 mg, 10%) (Sigma Aldrich), and polyvinylidenefluoride (PVDF, 2 mg, 10%) (Sigma-Aldrich) in 0.4 mL of N-methyl-2-pyrrolidone (NMP). Further, 12 μL of this slurry was spread onto the stainless steel (thickness = 0.06 mm) with an area of 1 cm2 and dried at 60 °C for overnight. An AC electrode was obtained, and the active material loading was (including acetylene black and PVDF) 4.0 ± 0.2 mg on each electrode.2.4. Fabrication of supercapacitor
A two-electrode test supercapacitor was fabricated with two identical AC electrodes and gel polymer electrolyte. The PVA–H2SO4–ARS gel polymer electrolyte membrane in the middle of two electrodes and assembled together by face-to-face. The gel polymer electrolyte membrane simultaneously served as both the electrolyte and the ion-porous separator, and the two stainless steel nets with the electrode material were used as current collectors. The fabrication model details of the supercapacitor are given in Fig. 1.2.5. Characterization
The mechanical performance of the redox-active mediated gel polymer was tested via bending, compressing and stretching. The gel polymer membrane was cut into a ribbon of 0.8 cm width and 3.0 cm length and the deformation was caused under appropriate forces.All electrochemical tests of two-electrode supercapacitors were performed at room temperature on an electrochemistry workstation (CHI660E, Shanghai Chenhua Co. Ltd, China). Cyclic voltammetry (CV) measurements were conducted in the potential range of 0–1.6 V at different scan rates of 5–50 mV s−1. The galvanostatic charge–discharge (GCD) behaviors were tested in the potential range of 0–1.6 V at different current densities from 0.5 to 3 A g−1. Electrochemical impedance spectroscopy (EIS) tests were carried out at frequencies ranging from 100 mHz to 100 kHz in open circuit potential. Moreover, the measurement of cycling stability were performed using computer controlled cycling equipment (LAND CT2001A, Wuhan, China).
The supercapacitor specific capacitance (C, F g−1) and electrode specific capacitance (Cs, F g−1) were evaluated from charge–discharge curves according to the following equation:24
 | (1) |
| Cs = 4C | (2) |
Energy density (E, W h kg−1) and power density (P, W kg−1) of the supercapacitor were calculated by the following equations:24
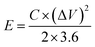 | (3) |
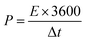 | (4) |
Besides, the ionic conductivity of gel polymer electrolyte in the supercapacitor was determined from the impedance spectrum. Gel polymer electrolyte was mounted on the conductivity holder with blocking stainless steel sheets as electrode. The ionic conductivity (σ, mS cm−1) of electrolyte can be calculated by the following equation:16
 | (5) |
The equivalent series resistance (ESR, Ω) of the supercapacitors with PVA–H2SO4 and PVA–H2SO4–ARS gel polymer electrolytes were evaluated by GCD and obtained from the following equations:25
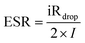 | (6) |
3. Results and discussion
3.1. Mechanical properties of the gel polymer electrolyte
The mechanical properties of a gel polymer electrolyte is a key factor in the application of corresponding flexible device. From Fig. 2a, it can be observed that the PVA–H2SO4–ARS gel polymer ribbon can be elastically stretched to more than 2 times of its length than its original, and return to the original dimensions after being stretched and released. As shown in Fig. 2b, a prepared cylinder of PVA–H2SO4–ARS gel polymer can be greatly compressed and return to the original dimensions. Moreover, the PVA–H2SO4–ARS gel polymer ribbon could be easily bent into a spiral or a circle without fracturing (Fig. 2c and d), and they can be quickly recover their original length and shape after the external force is removed, which indicated that the PVA–H2SO4–ARS gel polymer have excellent mechanical properties. Hence, the mechanical properties of PVA–H2SO4–ARS gel polymer can meet the fabrication of flexible devices.3.2. Ionic conductivity of gel polymer electrolyte
The ionic conductivity of gel polymer electrolyte affects the supercapacitor performance. The dependence of ARS amount on the ionic conductivity of gel polymer electrolyte is shown in Fig. 3. It is observed that the ionic conductivities gradually increase with the increase of ARS amount, and then gradually decrease with the further increment of their amount. The ionic conductivity of gel polymer electrolyte reaches to the highest value of 33.3 mS cm−1 at 0.50 g of ARS. This results indicated that the ionic conductivity of gel polymer electrolyte can be improved with the appropriate doping amount of ARS, which may be attributed to the ARS can act as plasticizer and redox shuttle in the electrolyte. However, when the ARS amount is less, ARS function as redox shuttle cannot realized well, and the conductivity of the electrolyte is smaller. Excessive amount of ARS will cause phase separation and association of free ions, which impeded the ions transport and induced the decrease of ionic conductivity of the gel polymer electrolyte.15,26 Therefore, 0.5 g of ARS is regarded as the optimal redox-mediated electrolyte amount in the PVA–H2SO4 gel polymer and used in further investigation.3.3. CV measurements
Fig. 4 shows the CV curves of supercapacitors with PVA–H2SO4 and PVA–H2SO4–ARS gel polymer electrolyte at a scan rate of 10 mV s−1. It's obvious that the supercapacitor with PVA–H2SO4 gel polymer electrolyte displays a rectangular shape, which is approximated to the ideal situation of electrical double layer capacitor,27 implying that charge and discharge occurred reversibly at electrode|electrolyte interface.20 When the redox-reactive ARS is added into the PVA–H2SO4 gel polymer electrolyte, a pair of remarkable and symmetric redox peaks appears in CV curves, which is attributed faradaic reactions occurring on the electrodes. These peaks are centered at 1.1 and 0.9 V corresponded to reversible hydrogenation–dehydrogenation reactions between the oxidation and reduction of ARS. The probably processes of redox reactions are presented in Scheme 1. Meanwhile, the appearance of the redox peaks indicates that the addition of ARS enhances electrode's capacitive performance, which is attributed to the co-existence of the double layer capacitance of porous carbon materials and pseudocapacitance contribution from the faradaic processes of redox mediator, and it can be inferred from the areas of CV curves.20,26 In addition, a distortion in the CV curves of supercapacitor with PVA–H2SO4 in high voltage since the electrolyte could be decomposed to hydrogen and/or oxygen.28 On the basis of the CV curves we can see that the supercapacitor based on the PVA–H2SO4–ARS electrolyte exhibits better electrochemical properties than the one utilizing the PVA–H2SO4 electrolyte, indicating ARS is available for the electrolyte systems. | ||
| Fig. 4 CV curves for the supercapacitors with PVA–H2SO4 and PVA–H2SO4–ARS gel polymer electrolytes at scan rate of 10 mV s−1. | ||
 | ||
| Scheme 1 Representation of the processes occurring on the carbon surface: double-layer formation and redox reaction. | ||
The CV curves of the supercapacitors with PVA–H2SO4–ARS gel polymer electrolyte were tested at different scan rates and are shown in Fig. 5a. It can be obviously observed that the series of CV curves shows outstanding current response over the potential window and the current density increased gradually with scan rate, indicating a surface-confined electrochemical process.29 The oxidation peaks moved positively and the reduction peaks moved negatively with the increase of the scan rates, which is mainly related to the internal resistance of the electrode.20
 | ||
| Fig. 5 (a) CV curves for the supercapacitor with PVA–H2SO4–ARS gel polymer electrolyte at different scan rates from 5 mV s−1 to 50 mV s−1. (b) Plots of peak currents vs. the square root of scan rate. | ||
Moreover, with the increase of the scan rates, the redox peaks tended towards gentle, suggested that the faradaic processes are restricted by rather slow diffusion and require time to trigger, particularly at a high scan rate. On the whole, the symmetry redox shape was maintained without significant distortion with the increasing potential scan rates even up to 50 mV s−1, demonstrating the PVA–H2SO4–ARS gel polymer electrolyte has reversible redox processes.
To further analyze CV characteristic of the supercapacitors with PVA–H2SO4–ARS gel polymer electrolyte, the responses of the oxidation and reduction peak currents to sweep rates (2–50 mV s−1) were measured. It can be seen from Fig. 5b that both the oxidation and reduction peak currents present a nearly linear relationship with the square root of the scan rate, proving that the redox reactions at the electrolyte/electrode interfaces correspond to quasi-reversible and diffusion-controlled processes. Moreover, it was found that the influence of the scan rate mainly had two effects: (1) the increase of the redox peak currents and (2) the increase in the peak potential separation when a sweep rate is increased. These behaviors confirm that both processes are diffusion controlled.30
3.4. Galvanostatic charge–discharge measurements
The capacitance performance of the supercapacitors with PVA–H2SO4 and PVA–H2SO4–ARS gel polymer electrolyte were investigated through GCD test at a current density of 1 A g−1 (Fig. 6a). The triangle behavior is observed for PVA/H2SO4 gel polymer electrolyte, which elucidates the ideal (or electric double layer) capacitive behavior.5 As the redox additives are incorporated into the gel polymer electrolyte, the nonlinear shape of the GCD curve prove the faradaic contributions to the charge accumulation process.27 The inclined parts in the charging potential and the discharging potential indicate that redox reactions are superimposed in the charge–discharge process,19 which are consistent with the cyclic voltammograms in Fig. 5a. Such characteristics similar to the previous reports,26,31 which are known to be typical effects of pseudocapacitive contributions. Furthermore, the discharge time of the supercapacitor based on PVA–H2SO4–ARS electrolyte is much longer than that with PVA–H2SO4 electrolyte, revealing great improvement in the electrochemical performances of the supercapacitor with PVA–H2SO4–ARS electrolyte. The longer charge–discharge time for the supercapacitor based on PVA–H2SO4–ARS electrolyte may be due to the additional contribution of a quick reversible redox process of ARS, which has been aforementioned.Moreover, the supercapacitors with PVA–H2SO4–ARS gel polymer electrolyte in two potential ranges exhibit a smaller iRdrop than the supercapacitor with PVA–H2SO4 gel polymer electrolyte, and the ESR are calculated as 6.25 and 11.88 Ω cm2, respectively. The ESR is the sum of the intrinsic resistance of all materials and the contact resistance between them. The above results indicate that the introduction of ARS improved the ionic conductivity of gel polymer electrolytes and enhanced conjunction between gel polymer electrolytes and electrodes via electron transfer between the mediators.20
The GCD curves of supercapacitors with PVA–H2SO4–ARS gel polymer electrolyte at different current densities are shown in Fig. 6b. According to the eqn (1), the specific capacitance of the supercapacitor based on PVA–H2SO4–ARS electrolyte is calculated as high as 441 F g−1 at a current density of 0.5 A g−1. Even though the current density is as high as 3 A g−1, the specific capacitance still maintains at a high value of about 235 F g−1 (Fig. 7a). Nevertheless the specific capacitance of the supercapacitor with the PVA–H2SO4 gel polymer is only 160 F g−1 at a current density of 0.5 A g−1. It indicates clearly that the supercapacitor with PVA–H2SO4–ARS gel polymer electrolyte shows superior electrochemical behavior compared with that of PVA–H2SO4 electrolyte.
 | ||
| Fig. 7 (a) Cs of supercapacitors with PVA–H2SO4–ARS gel polymer electrolyte at different current densities. (b) Ragone plots of supercapacitors with PVA–H2SO4–ARS gel polymer electrolyte. | ||
The energy density and power density are two key parameters of supercapacitors, which would limit the application of supercapacitors. The Ragone plots showing the dependence between power density and energy density are shown in Fig. 7b. It can be seen that the energy density decrease with the increase of power density. According to the eqn (4), the maximum energy density of supercapacitor based on PVA–H2SO4–ARS electrolyte can approach 39.4 W h kg−1 at a power density of 400 W kg−1. Even at a high power density of 2404.6 W kg−1, the energy density is still maintained at approximately 20.84 W h kg−1. Moreover, the obtained energy density are higher than that of previously reported flexible supercapacitors fabricated with gel polymer electrolyte.5,15,16,20,32 As a result, because of the redox reactions of ARS taking place on the electrode/electrolyte interfaces to give rise to the additional faradaic pseudocapacitances and increase the potential window, the energy density of supercapacitor is enhanced significantly. In addition, we connected our two series-connected cells based on PVA–H2SO4–ARS electrolyte to a red light-emitting diode (LED, 2.0 V) and easily lighted it after charged to 3.2 V for only 6 s (inset in Fig. 7b).
3.5. EIS measurements
Representative Nyquist impedance plots of the supercapacitors with PVA–H2SO4–ARS and PVA–H2SO4 gel polymer electrolytes are shown in Fig. 8. It can be seen that both plots contain semicircle at high frequency and straight line at the low frequency side along the imaginary axis, which is consistent with ideal electrochemical capacitance behavior.18 From the enlarged view of the higher frequency semicircles, it can be seen that the supercapacitor with PVA–H2SO4–ARS gel polymer electrolyte not only has lower inner resistance (Ri, 2.23 Ω cm2), calculated from the point of intersecting with the x-axis in the range of high frequency, but also has smaller charge transfer resistance (Rct, 0.70 Ω cm2), which was counted from the span of the single semicircle along the x-axis from high to low frequency. This indicates that the higher ionic conductivity and more smooth charges (electrons) and ions transfer for PVA–H2SO4–ARS system.33,34 Based on the above results, it can be concluded that the ARS mediator can enhance the interaction of electrode|electrolyte interface, which results in good electrochemical performance for PVA–H2SO4–ARS gel polymer electrolyte system. | ||
| Fig. 8 Nyquist plots of the supercapacitors with PVA–H2SO4–ARS and PVA–H2SO4 gel polymer electrolytes. | ||
3.6. Cycle life testing
Cyclic stability as an parameter to reflect the energy storage performance, is one of the most important electrochemical performances of supercapacitor. To investigate the cyclic stability, the galvanostatic charge/discharge cycling of the supercapacitor based on PVA–H2SO4–ARS electrolyte was performed at a current density of 1 A g−1 (Fig. 9). After 1000 cycles, the capacity can retain more than 78% of the initial capacitance. The observed capacitance loss can be ascribed to the intensive redox reactions introduced by the redox-active electrolytes and the incomplete redox reactions within the operating voltage window of the cell.27,35 In spite of that, the novel PVA–H2SO4–ARS electrolyte can be considered as a competitive and applicable electrolyte for high-performance flexible electrochemical devices. | ||
| Fig. 9 Cyclic performances of supercapacitors with PVA–H2SO4–ARS gel polymer electrolyte at a current density of 1 A g−1. | ||
4. Conclusion
A novel PVA–H2SO4–ARS gel polymer electrolyte were prepared by solution casting method and its feasibility was demonstrated by carbon based supercapacitors utilizing PVA–H2SO4–ARS as electrolytes. The ionic conductivity of gel polymer electrolytes is greatly increased with introducing of ARS and the sample containing 0.5 g of ARS exhibits an high conductivity of 33.3 mS cm−1 at 25 °C. Owing to the contribution of the redox reaction and quick reversible faradaic reactions of ARS, the supercapacitor with this redox-mediated electrolyte exhibits a large electrode specific capacitance of 441 F g−1, and a high energy density of 39.4 W h kg−1 at a current density of 0.5 A g−1. In addition, this device shows good cycle stability that maintains 78% of the initial capacitance values of after 1000 cycles. Therefore, this novel electrolyte has a good prospect for improving the electrochemical performance of an energy storage device, and can accelerate the development of application in other electrochemical fields.Acknowledgements
We thank the Science and Technology program of Gansu Province (no. 1308RJZA295, 1308RJZA265), the National Science Foundation of China (no. 21164009, 21174114), the program for Changjiang Scholars and Innovative Research Team in University (IRT1177), Lanzhou City University (LZCU-BS2013-11), Key Laboratory of Eco-Environment-Related Polymer Materials of Ministry of Education, and Key Laboratory of Polymer Materials of Gansu Province.References
- D. J. Lipomi, M. Vosgueritchian, B. C. Tee, S. L. Hellstrom, J. A. Lee, C. H. Fox and Z. Bao, Nat. Nanotechnol., 2011, 6, 788–792 CrossRef CAS PubMed
.
- M. Beidaghi and Y. Gogotsi, Energy Environ. Sci., 2014, 7, 867–884 CAS
.
- K. Qin, J. Kang, J. Li, C. Shi, Y. Li, Z. Qiao and N. Zhao, ACS Nano, 2015, 9, 481–487 CrossRef CAS PubMed
.
- K. Zhang, H. Hu, W. Yao and C. Ye, J. Mater. Chem. A, 2015, 3, 617–623 CAS
.
- S. T. Senthilkumar, R. K. Selvan, J. S. Melo and C. Sanjeeviraja, ACS Appl. Mater. Interfaces, 2013, 5, 10541–10550 CAS
.
- J. Xu, Q. Wang, X. Wang, Q. Xiang, B. Liang, D. Chen and G. Shen, ACS Nano, 2013, 7, 5453–5462 CrossRef CAS PubMed
.
- G. A. Tiruye, D. Muñoz-Torrero, J. Palma, M. Anderson and R. Marcilla, J. Power Sources, 2015, 279, 472–480 CrossRef
.
- Y. Zhu, S. Murali, M. D. Stoller, K. J. Ganesh, W. Cai, P. J. Ferreira and R. S. Ruoff, Science, 2011, 332, 1537–1541 CrossRef CAS PubMed
.
- H. Li, Q. Zhao, W. Wang, H. Dong, D. Xu, G. Zou and D. Yu, Nano Lett., 2013, 13, 1271–1277 CrossRef CAS PubMed
.
- X. Yang, F. Zhang, L. Zhang, T. Zhang, Y. Huang and Y. Chen, Adv. Funct. Mater., 2013, 23, 3353–3360 CrossRef CAS
.
- P. Yang, Y. Ding, Z. Lin, Z. Chen, Y. Li, P. Qiang and Z. L. Wang, Nano Lett., 2014, 14, 731–736 CrossRef CAS PubMed
.
- Q. Tang, M. Chen, G. Wang, H. Bao and P. Saha, J. Power Sources, 2015, 284, 400–408 CrossRef CAS
.
- B. H. Kim, K. S. Yang and J. P. Ferraris, Electrochim. Acta, 2012, 75, 325–331 CrossRef CAS
.
- J. Hao, Q. Xiao, G. Lei, Z. Li and L. Wu, Electrochim. Acta, 2014, 125, 450–456 CrossRef CAS
.
- J. Zhong, L. Q. Fan, X. Wu, J. H. Wu, G. J. Liu, J. M. Lin and Y. L. Wei, Electrochim. Acta, 2015, 166, 150–156 CrossRef CAS
.
- X. Zhang, L. Wang, J. Peng, P. Cao, X. Cai, J. Li and M. Zhai, Adv. Mater. Interfaces, 2015, 2 DOI:10.1002/admi.201500267
.
- S. T. Senthilkumar, R. K. Selvan and J. S. Melo, J. Mater. Chem. A, 2013, 1, 12386–12394 CAS
.
- H. Yu, J. Wu, L. Fan, K. Xu, X. Zhong, Y. Lin and J. Lin, Electrochim. Acta, 2011, 56, 6881–6886 CrossRef CAS
.
- H. Yu, J. Wu, L. Fan, Y. Lin, K. Xu, Z. Tang and Z. Lan, J. Power Sources, 2012, 198, 402–407 CrossRef CAS
.
- F. Yu, M. Huang, J. Wu, Z. Qiu, L. Fan, J. Lin and Y. Lin, J. Appl. Polym. Sci., 2014, 131 DOI:10.1002/app.39784
.
- M. Panizza and M. A. Oturan, Electrochim. Acta, 2011, 56, 7084–7087 CrossRef CAS
.
- J. E. Halls, S. D. Ahn, D. Jiang, L. L. Keenan, A. D. Burrows and F. Marken, J. Electroanal. Chem., 2013, 689, 168–175 CrossRef CAS
.
- G. Ma, M. Dong, K. Sun, E. Feng, H. Peng and Z. Lei, J. Mater. Chem. A, 2015, 3, 4035–4041 CAS
.
- L. Q. Fan, G. J. Liu, J. H. Wu, L. Liu, J. M. Lin and Y. L. Wei, Electrochim. Acta, 2014, 137, 26–33 CrossRef CAS
.
- C. Meng, C. Liu, L. Chen, C. Hu and S. Fan, Nano Lett., 2010, 10, 4025–4031 CrossRef CAS PubMed
.
- J. Wu, H. Yu, L. Fan, G. Luo, J. Lin and M. Huang, J. Mater. Chem., 2012, 22, 19025–19030 RSC
.
- S. Roldán, C. Blanco, M. Granda, R. Menéndez and R. Santamaría, Angew. Chem., Int. Ed., 2011, 50, 1699–1701 CrossRef PubMed
.
- H. Peng, G. Ma, K. Sun, J. Mu, Z. Zhang and Z. Lei, ACS Appl. Mater. Interfaces, 2014, 6, 20795–20803 CAS
.
- T. Kuila, A. K. Mishra, P. Khanra, N. H. Kim, M. E. Uddin and J. H. Lee, Langmuir, 2012, 28, 9825–9833 CrossRef CAS PubMed
.
- J. Xu, S. Gai, F. He, N. Niu, P. Gao, Y. Chen and P. Yang, J. Mater. Chem. A, 2014, 2, 1022–1031 CAS
.
- G. Wang, R. Liang, L. Liu and B. Zhong, Electrochim. Acta, 2014, 115, 183–188 CrossRef CAS
.
- M. K. Singh, M. Suleman, Y. Kumar and S. A. Hashmi, Energy, 2015, 80, 465–473 CrossRef CAS
.
- S. Rolda’n, M. Granda, R. Mene’ndez, R. Santamari’a and C. Blanco, J. Phys. Chem. C, 2011, 115, 17606–17611 Search PubMed
.
- H. Yu, L. Fan, J. Wu, Y. Lin, M. Huang, J. Lin and Z. Lan, RSC Adv., 2012, 2, 6736–6740 RSC
.
- W. Chen, C. Xia, R. B. Rakhi and H. N. Alshareef, J. Power Sources, 2014, 267, 521–526 CrossRef CAS
.
Footnote |
| † Feitian Ran and Kanjun Sun contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |




