Microwave-assisted synthesis of NaA nanozeolite from slag and performance of Ag-doped nanozeolite as an efficient material for determination of hydrogen peroxide†
Abstract
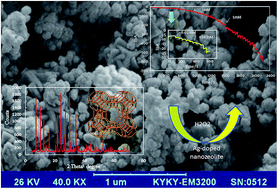
A new amperometric sensor is prepared based on a Ag doped NaA nanozeolite modified carbon paste electrode (Ag/ACPE) in order to detect hydrogen peroxide (H2O2) in phosphate buffer solution (PBS, pH 7.0). The NaA nanozeolite is synthesized from extracted silica of an industrial waste, silica slag, using microwave pretreatment and a hydrothermal method without organic additives. The sizes of NaA nanoparticles are in the range of 40 to 90 nm with an average diameter of 60 nm. The reduction current of H2O2 on Ag/ACPE is much higher than that on the non-modified NaA/CPE and CPE, which means the incorporation of Ag species into the NaA nanozeolite can create active sites for electrocatalytic reduction of H2O2. The Ag/ACPE sensor provides a linear response for H2O2 concentration with two linear ranges (0.02–7 mM and 7–30 mM) and has a low detection limit of 3.55 μM, a fast response time (t95% = 4 s), and a high sensitivity of about 651.37 μA mM−1 cm−2. Although oxygen interferes for H2O2 detection, the determination of peroxide in an anaerobic analyte cannot be affected by common interfering species such as ascorbic acid (AA), glucose, uric acid (UA) and sucrose. The obtained results show that the Ag modified zeolite has good performance as a sensor for H2O2 determination.

 Please wait while we load your content...
Please wait while we load your content...