Modeling the degradation and recovery of perovskite solar cells
Abstract
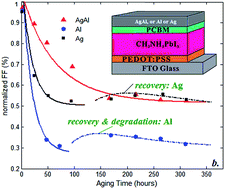
Degradation and recovery in the device parameters of perovskite-based solar cells are modeled for the devices aged by exposing to air humidity/moisture. Several devices are considered with the same CH3NH3PbI3−xClx absorber layer but with different windows, hole transport layers and metallic back contacts. We proposed several models to fit the experimental data, reported in the literature, on the degradation of fill factor, efficiency and short-circuit current density. The kinetics proposed for the modeling of degradation/recovery in the fill factor are based on variation in the fast and slow metastable defect states in the respective layer. The degradation/recovery of the short-circuit current density is investigated by proposing a new model which accounts for the density of metastable defects before/after aging. Finally, the degradation of the device efficiency is modeled by fitting the data with proper functions mostly following the Gaussian bi-exponential shape. It is shown that instability of the device parameters might be due to variation in metastable defects in any component of a perovskite solar cell. However, the fill factor, or consequently the series resistance, is the main degradation source in perovskite solar cells.

 Please wait while we load your content...
Please wait while we load your content...