Quantum interference effects in biphenyl dithiol for gas detection†
Jariyanee
Prasongkit
*ab and
Alexandre R.
Rocha
c
aDivision of Physics, Faculty of Science, Nakhon Phanom University, Nakhon Phanom 48000, Thailand. E-mail: jariyanee.prasongkit@npu.ac.th
bNanotec-KKU Center of Excellence on Advanced Nanomaterials for Energy Production and Storage, Khon Kaen 40002, Thailand
cInstituto de Física Teórica, Universidade Estadual Paulista (UNESP), São Paulo, SP, Brazil
First published on 15th June 2016
Abstract
Based on density functional theory and non-equilibrium Green's function method, we investigate the transport properties of four different gases (NO, NO2, NH3, and CO) adsorbed on biphenyl dithiol molecules coupled to gold leads. Since the transmission function is strongly affected by quantum interference, namely antiresonance and Fano resonances, significant changes in conductance caused by the adsorption of molecular gases are archived. Our findings suggest that biphenyl dithiol exhibits strong sensitivity for NO and NO2 which results from their interference features being close to the Fermi energy of the gold leads and dominate the transport of the system. Moreover, the quantum interference effects also result in enhanced and suppressed conductance, which enable us to significantly improve the selectivity. In particular, a distinction between NO, NO2 and NH3 gases based on the biphenyl dithiol appears possible.
1 Introduction
Charge transport in metal–molecule–metal junctions is of fundamental interest since such junctions represent a significant step toward the understanding of quantum transport theories1,2 and pave the way towards possible applications3 in nanoelectronics. Many phenomena in single molecules connected to nano-gap electrodes: e.g. giant magnetoresistance,4 conductance switching,5–8 negative differential resistance,9–11 have been observed. Additionally, theorists have predicted that it should be possible to observe quantum interference in single molecules,12–14 which is a characteristic of coherent transport. As previously discussed,15–20 quantum interference can control electrical transport, leading to the possibility of modulating the conductance through molecular conformation, charge distribution and frontier molecular orbitals, and gives us opportunities for more advanced molecular electronic components.Experimentally, it has been demonstrated that quantum interference in molecular junctions could be measured at room temperature.16–20 On the other hand, there are only few reports where interference-based devices have been proposed; for example, H–H tautomerization molecular switch studied by us,21 quantum interference effect transistors22 as well as quantum interferences in DNA sequencing.23,24 So far, to the best of our knowledge, no studies have demonstrated applications as gas sensor devices based on quantum interference. Over the last few years, several nanoscale gas sensors have been developed utilizing nanostructured materials; for instance, carbon nanotubes,25,26 graphene,27,28 silicene29 and other layered nanomaterials.30,31 Moreover, it has been suggested that organic materials, such as metal porphyrin32,33 or phthalocyanine,34 can be used to detect various gases with high sensitivity and selectivity due to a direct modification of their molecular orbital states as well as their unique properties of molecular recognition.
Several theoretical and experimental studies have been previously reported concerning single-molecule biphenyl dithiol (BPDT) junctions for the possibility of future applications in nanoelectronics such as molecular switches based on conformational change35–38 and thermoelectricity in molecular junctions.39,40 Particularly in the latter case,40 it was experimentally demonstrated that the electrical conductance of molecular junctions can be controlled by a gate electrode. A few theoretical studies37,38,41,42 based on a combination of the non-equilibrium Green's function (NEGF) technique and density functional theory (DFT) were carried out to investigate the electronic structure and transport properties of BPDT. However, to our knowledge, there is no study on a quantum interference sensor device based on BPDT. At least one DFT-based study43 has demonstrated quantum interference effects in Au–S–BPDT–S–Au junctions, resulting from different molecular bridge sites between the BPDT and electrodes.
The purpose of our study was to investigate the quantum interference effects of BPDT with gas adsorption to assess the usefulness of this setup for the purpose of gas sensing. We study the transport properties of BPDT coupled to two Au(111) surfaces using a combination of NEGF techniques44–46 and DFT.47 Four gas molecules; i.e., NO, NO2, NH3, and CO, which are all of main interest for industrial, environmental and medical purposes, were adsorbed onto BPDT. The results indicate changes in conductance due to interference features in the electronic transmission. The possibility to discriminate four different gas molecules (NO, NO2, NH3, and CO) has been discussed in terms of sensitivity and selectivity.
2 Methodology
The molecular structure of BPDT, whose S atoms were terminated with H atoms, was first optimized. The obtained bond lengths and molecular configuration are similar to previous DFT studies.41,42,48 To construct the BPDT molecular device, the molecule was placed between semi-infinite Au(111)-(3 × 3) electrodes (see Fig. 1a). Such a system was divided into three regions: left and right electrodes and a central region. The central region contains a portion of the electrode to screen out any perturbation of the metal coming from BPDT. The molecular adsorption of a thiol was determined by placing BPDT above different adsorption sites: on top, bridge, fcc and hcp, on Au(111) surface. The BPDT is adsorbed at a hollow site at which the average distance between the S atom and the two nearest neighbor Au atoms is 2.53 Å. The device configuration with two electrodes was then optimized again, allowing all atoms in the scattering region to relax.All optimizations were performed using DFT,49,50 as implemented in the SIESTA package.47 We employed a van der Waals correction51,52 taking into account the weak dispersive interactions to the generalized gradient approximation of Perdew, Burke, and Ernzerhof (PBE–GGA).53
A description of van der Waals (vdW) interactions was included in our calculations using the non-local van der Waals density functional (vdW-DF) of Dion et al.54 In this approach, the exchange–correlation energy is written as Exc = EGGAx + ELDAc + Enlc, where the exchange energy EGGAx uses the GGA functional, and ELDAc is the local density approximation (LDA) to correlation energy. The nonlocal correlation-energy part, Enlc, is defined to include the longest-ranged or most nonlocal energy term which is zero for systems with constant density.
A double-ζ plus polarization (DZP) basis and norm-conserving pseudopotentials55 were employed. The k-point was set to 5 × 5 × 3 for the Brillouin zone integration. The mesh cutoff value was 350 Ry for the real-space grid. The binding energy between the BPDT and the gas molecules was calculated as (EBPDT + Egas) − Etotal, where Etotal is the total energy of the system (BPDT + gas), and EBPDT and Egas are the energy of the BPDT and gas, respectively.
The transport properties were investigated by the quantum transport code TranSIESTA, with the NEGF method. Since TranSIESTA uses the SIESTA code as its DFT platform, the basis sets and real-space grid employed are the same as those used for the geometrical relaxation part. Within the NEGF approach, the transmission indicating the probability for electrons with incident energy E to be transferred from the left to the right electrode, is calculated from T(E) = Tr[ΓR(E)GR(E)ΓL(E)GA(E)], where ΓL,(R)(E) is the broadening matrix of the left (right) electrode, and GR,(A)(E) is the retarded (advanced) Green's function.45 We simply evaluated the conductance, G = G0T(EF),56 where  is the quantum conductance, e is the charge of the electron, and h is Planck's constant. Throughout this paper, it should be noted that we will focus on zero-bias transmission, in which the pronounced interference features appear and are the most straightforward to understand.
is the quantum conductance, e is the charge of the electron, and h is Planck's constant. Throughout this paper, it should be noted that we will focus on zero-bias transmission, in which the pronounced interference features appear and are the most straightforward to understand.
3 Results
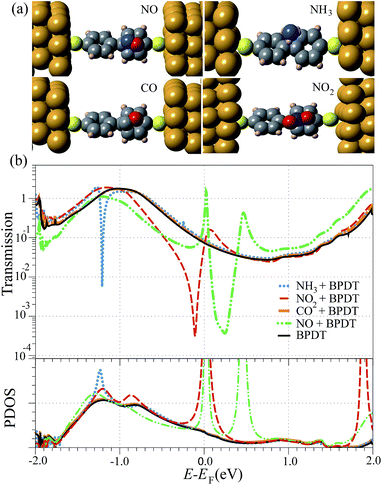
Fig. 1a shows the most favorable adsorption configurations of the NO2, NO, NH3 and CO molecules on the BPDT. Different molecular orientations of the gases were initially placed at different positions on the BPDT molecule (see more details in the ESI†). The C and N atoms of CO and NO adsorbed on the ring-connecting C atom of the BPDT molecule with a binding distance of 3.02 Å and 2.52 Å, respectively, while five non-connecting C atoms become less stable adsorption sites. Note that the binding distance is defined as the shortest atom-to-atom distance between the BPDT and the gas molecules. Similarly, NO2 prefers binding to the ring-connecting C atom with a binding distance of 2.50 Å. In the case of NH3, its three hydrogen atoms pointing toward the BPDT were found to be the most favorable configuration with a binding distance of 3.11 Å. The NH3 has also a tendency to lie above the ring-connecting C atom, however, the binding energies of NH3 adsorbed on each site of the BPDT are slightly different due to a weak interaction between them. The binding energies of NO2, NO, NH3 and CO adsorbed on the BPDT were found to be 0.17, 0.14, 0.09, and 0.07 eV, respectively. The binding energies of NH3 and CO adsorbed on the BPDT are rather weak, whereas for the NO and NO2 the interaction is stronger. This is due to the fact that the presence of one unpaired electron makes the latter molecules chemically reactive toward BPDT. Mulliken population analysis indicates that the BPDT acts as an electron donor, and the charge transfer from BPDT to NO2, NO, NH3 and CO is found to be 0.097|e|, 0.02|e|, 0.015|e| and 0.01|e|, respectively. The adsorption energies and charge transfer suggest that gas molecules are physisorbed on BPDT.Fig. 1b presents the transmission T(E) of the NO, NO2, CO and NH3 molecules adsorbed on the BPDT devices (top panel) and the Projected Density of States (PDOS) for atoms in the central region (bottom panel). The black solid line corresponds to the reference transmittance without the presence of gas molecules. Let us first discuss the T(E) of BPDT without gas adsorption. The HOMO level of BPDT, which gives rise to a broad resonance peak below the Fermi energy (E − EF ∼ −1 eV), plays the dominant role in the electron transport of BPDT, whereas the LUMO energy level is further away from the Fermi energy. Comparing the transport properties of BPDT–Au junction with previously reported results37,38,41,42 using the NEGF-DFT approach, the main features of PDOS and T(E) are very similar. A variation in the transmission peak height could be due to the difference between exchange–correlation functionals, energy cutoff and number of k-points. When compared the T(E) and the PDOS peaks, we obtained a good agreement. The broad PDOS peak below the Fermi energy indicates the strong interaction between the HOMO of BPDT and the electrodes. The HOMO mainly arises from a π-type molecular orbital, which compares well with several previous studies.41,42,57
Except in the case of CO, the transmission was strongly affected (see Fig. 1b) when the gas molecules were adsorbed on BPDT. A sharp rise in the PDOS suggests that the coupling between the electronic states of the gas and the electrodes is weak. As a result, the discrete energy states of NO, NO2 and NH3 couple to the continuum electronic states of BPDT, leading to pronounced interference effects in the window around EF.
The signature of quantum interference, appearing in the transmission, needs to be addressed in details. In the case of NH3, the sharp dip in T(E) appears at ∼−1.21 eV below the Fermi energy indicating antiresonance. To clarify the origin of the observed quantum interference, we investigated the local currents of NH3 adsorbed on the BPDT around the interference feature; i.e., −1.22 eV and −1.20 eV. These are shown in Fig. 2a, left panel; the arrows point the direction of net current flow from the left to the right electrode. For NH3, we could observe the multi-path quantum interference, which exhibits the suppression at one of the current paths. The antiresonance can be characterized by the reversal of ring currents.58 For BPDT without gas adsorption, a previous study43 has demonstrated that large transmission near the Fermi energy results from constructive contributions from HOMO and LUMO. As seen in Fig. 2a (right), two paths of the local currents around the ring at a specific energy; i.e. E − EF = −1.00 eV, constructively interact, which are consistent with those previous results.43
The transmitting eigenchannel wavefunctions from the left to the right electrode46 were investigated to provide a clear physical picture to help us understand the interference features characterizing the transmission, as presented in Fig. 2b. For NH3 adsorbed on BPDT, it is evident that the behavior on either side of the orbital wave function differs; the sign of the orbital wave functions is reversed above the dip (area 2 shaded in orange), whereas for BPDT without gas this reversal in sign of the wave function wasn't observed (area 3 shaded in gray). The interference feature in this case, therefore, arises from destructive quantum interference, in which the wave functions propagate through different spatial quantum paths in the π-system of BPDT backbone.
Another type of quantum interference in the transmission: i.e., Fano resonance, was observed around the Fermi energy for the NO and NO2, as shown in Fig. 1b. The origin of Fano resonance arises from the constructive and destructive interference of a narrow discrete resonance with a broad spectral line or continuum.15,59,60 In our study, PDOS peaks indicate the strong coupling of BPDT–electrode and weak coupling for gas molecules since the PDOS peak widths of the former case are broader. As a result, the Fano resonance arises from coupling between the discrete energy states of the NO and NO2 and the continuum of states supplied by the BPDT–electrode (see ESI, Fig. S2†). A dip and a peak in the transmission spectra around the Fermi energy are in agreement with the Fano resonance characteristics.
For NO, the Fano resonance gives rise to the transmission peak close to the Fermi energy whose scattering state with π character (the area 5 shaded in red) is localized along the backbone of the BPDT. The eigenchannel wavefunctions shown were the ones below and above the Fermi level (area 4 and 6 shaded in yellow and pink), showing the exponential decay of the π electron state. Particularly, we noticed that the sign of the orbital wave functions of BPDT is reversed above the transmission peak (area 4 shaded in pink). Similarly, the Fano resonance induced by NO2 causes the dip and the peak in the transmission close to the Fermi level, and the phase reversal of the eigenchannels was also observed just above transmission dip (area 8 shaded in green).
Next, we turn our attention to the capability of BPDT as a gas sensor to distinguish four different gas molecules (NO, NO2, NH3 and CO). The performance of this sensor device would be discussed in terms of sensitivity and selectivity to various gases. Electron transmission changes around the Fermi level results in a variation in conductance. The sensitivity can be defined as  , where G(T) and G0(T0) are the zero bias conductance (transmission) for a gas sensor with and without gas adsorption, respectively. Furthermore, it may be possible to improve the selectivity; the quantum interference effects can be tuned by applying a gate voltage to the system,61 leading to a change in the position and the dip and peak features. The conductance for a small gate voltage can be defined as
, where G(T) and G0(T0) are the zero bias conductance (transmission) for a gas sensor with and without gas adsorption, respectively. Furthermore, it may be possible to improve the selectivity; the quantum interference effects can be tuned by applying a gate voltage to the system,61 leading to a change in the position and the dip and peak features. The conductance for a small gate voltage can be defined as  , where the μ = EF − eVg is the chemical potential.
, where the μ = EF − eVg is the chemical potential.
The sensitivity as a function of the gate voltage was determined, as shown in Fig. 3. Note that positive and negative sensitivities in Fig. 3 indicate the predicted enhanced and suppressed conductance, respectively. At Vg = 0 V, the interference feature of NO and NO2 is very close to the Fermi energy and dominates the transport of the system, whereas those of NH3 are far from the Fermi energy. Nevertheless, the sensitivity histograms exhibit a distinction between NO, NO2 and NH3 appears possible with a zero-bias voltage applied. The positive of NH3 and negative values of NO2 indicate the predicted enhanced and suppressed conductance, respectively. The absolute values of sensitivities were found to follow an ascending order; i.e., CO ≪ NH3 ∼ NO2 ≪ NO. The very large sensitivity of NO adsorbed on BPDT enables us to distinguish it from others, while the NH3 and NO2 adsorbed on BPDT shows a reversal in conductance allowing for the electrical distinction between them. At Vg = −1.2, −0.1 and 0.2 V, the BPDT shows the sensitivity towards NO with decreasing conductance, as opposed to large increasing conductance at Vg = 0 V. For NO2, the conductance at Vg = 0.2 and −0.1 V is shown to be increased and decreased, respectively. For NH3, the decrease in conductance was observed only at Vg = −1.2. Hence, applying the gate voltages to this setup should help to distinguish between different gas molecules.
4 Conclusions
In summary, we investigated the quantum interference effects in the tunneling conductance of BPDT-molecules adsorption, connected to gold leads. We employed first-principles methods based on DFT formalism to determine the favorable adsorption configurations, binding energies and charge transfer of gas molecules (NO, NO2, NH3, and CO) on BPDT. Our results indicate that NH3 adsorbed on BPDT exhibits destructive quantum interference giving rise an antiresonance in the transmission, while Fano resonance features in the transmission were observed for NO and NO2. Based on our results, a BPDT device is capable of detecting different gas species with high sensitivity and selectivity. The findings reported by us here can be considered important enough to experimentalists who might be encouraged by our positive results to consider our proposed design and take on the challenging task of fabricating this setup to put our predictions to the test of gas sensing through direct measurements.Acknowledgements
J. P. acknowledges the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, the Thailand Research Fund (MRG5980185), and the National Nanotechnology Center (NANOTEC), NSTDA, Ministry of Science and Technology, Thailand, through its program of Centers of Excellence Network. A. R. R. would like to acknowledge Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).References
- A. Nitzan and M. A. Ratner, Science, 2003, 300, 1384–1389 CrossRef CAS PubMed.
- S. Datta, Electron Transport in Mesoscopic Systems, Cambridge University Press, Cambridge, 1995 Search PubMed.
- N. J. Tao, Nat. Nanotechnol., 2006, 1, 173–181 CrossRef CAS PubMed.
- S. Schmaus, A. Bagrets, Y. Nahas, T. K. Yamada, A. Bork, M. Bowen, E. Beaurepaire, F. Evers and W. Wulfhekel, Nat. Nanotechnol., 2011, 6, 185–189 CrossRef CAS PubMed.
- S. J. van der Molen and P. Liljeroth, J. Phys.: Condens. Matter, 2010, 22, 133001 CrossRef PubMed.
- J. Prasongkit, A. Grigoriev and R. Ahuja, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 155434 CrossRef.
- B. Standley, W. Bao, H. Zhang, J. Bruck, C. N. Lau and M. Bockrath, Nano Lett., 2008, 8, 3345–3349 CrossRef CAS PubMed.
- Q. Fu, J. Yang and Y. Luo, Appl. Phys. Lett., 2009, 95, 182103 CrossRef.
- M.-Q. Long, K.-Q. Chen, L. Wang, W. Qing, B. S. Zou and Z. Shuai, Appl. Phys. Lett., 2008, 92, 243303 CrossRef.
- H. S. Majumdar, J. K. Baral, R. Österbacka, O. Ikkala and H. Stubb, Org. Electron., 2005, 6, 188–192 CrossRef CAS.
- Y. Karzazi, J. Cornil and J. L. Bredas, J. Am. Chem. Soc., 2001, 123, 10076–10084 CrossRef CAS PubMed.
- P. Sautet and C. Joachim, Chem. Phys. Lett., 1988, 153, 511–516 CrossRef CAS.
- D. Walter, D. Neuhauser and R. Baer, Chem. Phys., 2004, 299, 139–145 CrossRef CAS.
- D. Q. Andrews, G. C. Solomon, R. P. V. Duyne and M. A. Ratner, J. Am. Chem. Soc., 2008, 130, 17309–17319 CrossRef CAS PubMed.
- C. J. Lambert, Chem. Soc. Rev., 2015, 44, 875–888 RSC.
- C. M. Guédon, H. Valkenier, T. Markussen, K. S. Thygesen, J. C. Hummelen and S. J. van der Molen, Nat. Nanotechnol., 2012, 7, 305–309 CrossRef PubMed.
- H. Vazquez, R. Skouta, S. Schneebeli, M. Kamenetska, R. Breslow, L. Venkataraman and M. S. Hybertsen, Nat. Nanotechnol., 2012, 7, 663–667 CrossRef CAS PubMed.
- S. Ballmann, R. Härtle, P. B. Coto, M. Elbing, M. Mayor, M. R. Bryce, M. Thoss and H. B. Weber, Phys. Rev. Lett., 2012, 109, 056801 CrossRef PubMed.
- V. Kaliginedi, P. Moreno-Garca, H. Valkenier, W. Hong, V. M. Garca-Surez, P. Buiter, J. L. H. Otten, J. C. Hummelen, C. J. Lambert and T. Wandlowski, J. Am. Chem. Soc., 2012, 134, 5262–5275 CrossRef CAS PubMed.
- S. V. Aradhya and L. Venkataraman, Nat. Nanotechnol., 2013, 8, 399–410 CrossRef CAS PubMed.
- J. Prasongkit, A. Grigoriev, R. Ahuja and G. Wendin, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 165437 CrossRef.
- C. A. Stafford, D. M. Cardamone and S. Mazumdar, Nanotechnology, 2007, 18, 424014–424017 CrossRef PubMed.
- H. Jeong, H. S. Kim, S. H. Lee, D. Lee and Y. H. Kim, Appl. Phys. Lett., 2013, 103, 023701 CrossRef.
- S. K. Min, W. Y. Kim, Y. Cho and K. S. Kim, Nat. Nanotechnol., 2011, 6, 1–4 CrossRef PubMed.
- R. G. Amorim, A. Fazzio, A. J. R. da Silva and A. R. Rocha, Nanoscale, 2013, 5, 2798–2803 RSC.
- J. Li, Y. Lu, Q. Ye, M. Cinke, J. Han and M. Meyyappan, Nano Lett., 2003, 3, 929–933 CrossRef CAS.
- J. D. Fowler, M. J. Allen, V. C. Tung, Y. Yang, R. B. Kaner and B. H. Weiller, ACS Nano, 2009, 3, 301–306 CrossRef CAS PubMed.
- F. Schedin, A. K. Geim, S. V. Morozov, E. W. Hill, P. Blake, M. I. Katsnelson and K. S. Novoselov, Nat. Mater., 2007, 6, 652–655 CrossRef CAS PubMed.
- J. Prasongkit, R. G. Amorim, S. Chakraborty, R. Ahuja, R. H. Scheicher and V. Amornkitbamrung, J. Phys. Chem. C, 2015, 119, 16934–16940 CrossRef CAS.
- L. Kou, T. Frauenheim and C. Chen, J. Phys. Chem. Lett., 2014, 5, 2675–2681 CrossRef CAS PubMed.
- B. Cho, M. G. Hahm, M. Choi, J. Yoon, A. R. Kim, Y.-J. Lee, S.-G. Park, J.-D. Kwon, C. S. Kim, M. Song, Y. Jeong, K.-S. Nam, S. Lee, T. J. Yoo, C. G. Kang, B. H. Lee, H. C. Ko, P. M. Ajayan and D.-H. Kim, Sci. Rep., 2015, 5, 8052 CrossRef CAS PubMed.
- H. Kondo, J. Nara and T. Ohno, J. Phys. Chem. C, 2011, 115, 6886–6892 CrossRef CAS.
- N. Wang, H. Liu, J. Zhao, Y. Cui, Z. Xu, Y. Ye, M. Kiguchi and K. Murakoshi, J. Phys. Chem. C, 2009, 113, 7416–7423 CrossRef CAS.
- F. I. Bohrer, C. N. Colesniuc, J. Park, M. E. Ruidiaz, I. K. Schuller, A. C. Kummel and W. C. Trogler, J. Am. Chem. Soc., 2009, 131, 478–485 CrossRef CAS PubMed.
- A. Mishchenko, D. Vonlanthen, V. Meded, M. Bürkle, C. Li, I. V. Pobelov, A. Bagrets, J. K. Viljas, F. Pauly, F. Evers, M. Mayor and T. Wandlowski, Nano Lett., 2010, 10, 156–163 CrossRef CAS PubMed.
- M. E. Z. Michoff, M. E. Castillo and E. P. M. Leiva, J. Phys. Chem. C, 2013, 117, 25724–25732 CrossRef CAS.
- M. Bürkle, J. K. Viljas, D. Vonlanthen and A. Mishchenko, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 075417 CrossRef.
- F. Pauly, J. K. Viljas, J. C. Cuevas and G. Schön, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 155312–155319 CrossRef.
- N. Sergueev, S. Shin, M. Kaviany and B. Dunietz, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 195415–195512 CrossRef.
- Y. Kim, Nat. Nanotechnol., 2014, 9, 881–885 CrossRef CAS PubMed.
- H. Kondo, J. Nara, H. Kino and T. Ohno, J. Chem. Phys., 2008, 128, 064701–064708 CrossRef PubMed.
- H. Kondo, J. Nara, H. Kino and T. Ohno, J. Phys.: Condens. Matter, 2009, 21, 064220 CrossRef PubMed.
- N. Gorczak, N. Renaud, S. Tarkuç, A. J. Houtepen, R. Eelkema, L. D. A. Siebbeles and F. C. Grozema, Chem. Sci., 2015, 6, 4196–4206 RSC.
- M. Brandbyge, J.-L. Mozos, P. Ordejón, J. Taylor and K. Stokbro, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 65, 165401 CrossRef.
- Y. Xue, S. Datta and M. A. Ratner, Chem. Phys., 2002, 281, 151–170 CrossRef CAS.
- M. Paulsson and M. Brandbyge, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 115117 CrossRef.
- M. Soler, E. Artacho, J. D. Gale, A. Garc, J. Junquera, P. Ordej and S. Daniel, J. Phys.: Condens. Matter, 2002, 2745, 2745–2779 CrossRef.
- M. Mamatkulov, L. Stauffer, C. Minot and P. Sonnet, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 035321–035327 CrossRef.
- P. Hohenberg and W. Kohn, Phys. Rev., 1964, 155, 864–870 CrossRef.
- W. Kohn and L. Sham, Phys. Rev., 1965, 385, 1133–1138 CrossRef.
- M. Dion, H. Rydberg, E. Schröder, D. C. Langreth and B. I. Lundqvist, Phys. Rev. Lett., 2004, 92, 246401 CrossRef CAS PubMed.
- G. Román-Pérez and J. Soler, Phys. Rev. Lett., 2009, 103, 096102 CrossRef PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- M. Dion, H. Rydberg, E. Schröder, D. C. Langreth and B. I. Lundqvist, Phys. Rev. Lett., 2004, 92, 246401 CrossRef CAS PubMed.
- N. Troullier, Phys. Rev. B: Condens. Matter Mater. Phys., 1991, 43, 1993–2006 CrossRef CAS PubMed.
- D. S. Fisher and P. A. Lee, Phys. Rev. B: Condens. Matter Mater. Phys., 1981, 23, 6851–6854 CrossRef CAS.
- R. Cohen, K. Stokbro, J. M. L. Martin and M. A. Ratner, J. Phys. Chem. C, 2007, 111, 14893–14902 CrossRef CAS.
- G. C. Solomon, C. Herrmann, T. Hansen, V. Mujica and M. A. Ratner, Nat. Chem., 2010, 2, 223–228 CrossRef CAS PubMed.
- A. E. Miroshnichenko, S. Flach and Y. S. Kivshar, Rev. Mod. Phys., 2010, 82, 2257–2298 CrossRef CAS.
- B. Luk’yanchuk, N. I. Zheludev, S. A. Maier, N. J. Halas, P. Nordlander, H. Giessen and C. T. Chong, Nat. Mater., 2010, 9, 707–715 CrossRef PubMed.
- G. C. Solomon, D. Q. Andrews, R. H. Goldsmith, T. Hansen, M. R. Wasielewski, R. P. Van Duyne and M. A. Ratner, J. Am. Chem. Soc., 2008, 130, 17301–17308 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra06578c |
| This journal is © The Royal Society of Chemistry 2016 |