HKUST-1 silica aerogel composites: novel materials for the separation of saturated and unsaturated hydrocarbons by conventional liquid chromatography†
Abstract
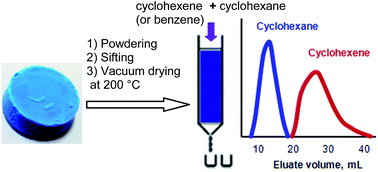
HKUST-1 silica aerogel composite (HKUST-1@SiO2) has been studied as a stationary phase for the efficient separation of unsaturated hydrocarbons from saturated aliphatics by conventional liquid chromotography (LC). HKUST-1@SiO2 has been prepared via an advanced sol–gel method and subsequent drying in supercritical CO2 to minimize the deterioration of the individual properties of the MOF and silica aerogel structures and tune its properties to be acceptable for flow mode. The synthesized composite was characterized by X-ray diffraction, FT-IR spectroscopy, XPS, scanning electron microscopy with EDAX mapping and low-temperature nitrogen adsorption. According to these data, the composite represents physically dispersed domains of HKUST-1 in the silica aerogel network. It was shown that the HKUST-1@SiO2 composite can be used as a highly efficient stationary phase for conventional liquid chromatographic separation of cyclohexene or benzene from cyclohexane. This is the first time a MOF composite has been used for the separation of organic molecules by LC, demonstrating new vistas for the application of these materials in the flow mode.

 Please wait while we load your content...
Please wait while we load your content...