DOI:
10.1039/C6RA06498A
(Paper)
RSC Adv., 2016,
6, 46900-46907
Synthesis of polypyrrole–polystyrene composite microspheres via pseudo-multicomponent heterophase polymerization and the potential application on Cr(VI) removal
Received
11th March 2016
, Accepted 30th April 2016
First published on 3rd May 2016
Abstract
Polypyrrole–polystyrene (PPy–PS) composite hollow microspheres with a dry-plum-like morphology were prepared via pseudo-multicomponent heterophase polymerization in an aqueous dispersion system. The monomers of pyrrole (Py) and styrene (St) were simultaneously added into the reactor with the existence of azodiisobutyronitrile. The oxidization polymerization of Py was first initiated by the addition of ammonium persulfate, and the radical polymerization of St was subsequently initiated by the elevated temperature. Finally, composite micron-sized spheres were obtained with hierarchical structures. The microspheres are hollow inside, with PPy nano-particles bound by a PS matrix for the building of the wall-shell. The decoration of tiny PPy particles at the surface leads the microspheres to be an effective adsorbent to isolate Cr(VI) ions from water. The adsorption kinetics and isotherm result of the microspheres agree well with the pseudo-second-order rate model and Langmuir isotherm mode, respectively.
Introduction
With the development of modern industry, the contamination of heavy metal ions has been a serious problem.1 Many techniques have been developed to remove heavy metal ions from water, such as electrochemical reduction, electro-coagulation, ion exchange, membrane separation, and adsorption.2 Among them, the adsorption technique has caused extensive concern. In recent years, polypyrrole (PPy) has been found to be a satisfactory absorbent material with the controllable reversibility and the high activity to bind with heavy metal ions.3
Ansari et al. reported that PPy-coated wood sawdust can be used as an efficient absorbent to remove Cr(VI) ions from water.4 Yao et al. demonstrated that PPy-coated palygorskite has a great potential application in the elimination of anionic contaminations from wastewater.5 It should be noted that the state of the absorbent can affect the separation efficiency. Due to the tremendous specific area, PPy-based nanoparticles (NPs) show a higher absorption capability, compared with its bulk and films.6 However, in contrast with the widely applied microspheres in separation operations, such as column separation, the application of NPs is limited by the difficulty in manipulation and the strong aggregation tendency.
One of the methods to resolve the problems is to build the microspheres with hierarchical structure. By binding the functional NPs onto the micron-sized spheres, the large sphere matrix will provide a great convenience for the direct application of PPy-based composites. However, the building of such hierarchical spheres can be complicated. In a typical synthesis, the template (organic particle or inorganic particle) is necessary, whose surface should be chemically or physically modified to ensure the affinity with the subsequently coated functional polymers or NPs.7 Additionally, due to the characteristics of large surface area, low density, structural stability, hollow particles have been much concerned. The voids inside them make us possible get maximum specific surface area by using the raw materials as little as possible, so as to improve the adsorption performance with low consumption. If the target product is hollow spheres, the synthesized core–shell composites have to be treated by solvent extraction or high temperature calcinations to remove the templates. Lu et al. prepared Fe3O4–PPy core–shell particles by such method.8
To simplify the synthetic operation, one of the inventions is the multicomponent reaction (MCR), which permits the “one-pot” preparation via the combination of simultaneous reactions or the sequential addition procedure without changing solvent. The application of MCR in polymerization system has also been explored in the building of polymeric materials with charming morphologies and high efficient functionalities.9 According to the previous investigation, we suppose that it would also be possible to prepare PPy-based composites with well-defined hierarchical structures via a one-pot approach in a multicomponent system. To distinguish from the traditional MCR in organic synthesis, we name the synthesis method in this work as pseudo-multicomponent heterophase polymerization (PMHP): the key for the fabrication of the nanocomposites should be the in situ interfacial anchoring on the simultaneously or subsequently generated intermediate.10–12
Nowadays, many researches on the emulsion and dispersion have been reported, which are stabilized by tiny solid particles (named as Pickering particles or emulsifiers).13 Poly(3,4-ethylenedioxythiophene) (PEDOT) has been reported as the Pickering emulsifier in emulsion polymerization to prepare conductive composite latexes.14 The result implies that the pre-formed conducting polymer particles could assemble at polymer/water interface in a heterophase polymerization system. In our PMHP synthesis with pyrrole (Py) for oxidation polymerization and styrene (St) for radical polymerization added in one pot in aqueous dispersions, we sequentially initiated the two polymerizations. The first initiated polymerization of Py produced PPy, which is deposited out as tiny particles with the presence of the proper stabilizer. We expect to employ the in situ formed PPy particles as Pickering emulsifier to stabilize the following polymerization of St. Meanwhile, the polymerization of Py at the interface would further proceed, based on the step-polymerization mechanism. The combination of these two polymerizations in one-pot produced composite hollow microspheres with dry-plum-like morphology (diameter around 3 μm). The wall of the spheres is composed of nano-structured PPy particles, which are tightly bound by PS. Furthermore, the PPy–PS composites were used to remove Cr(VI) ions from water. The adsorption property of the microspheres was also systematically explored in this work.
Experimental section
Materials
St, Py(99+%, distilled under reduced pressure), ammonium persulfate (APS), tetrahydrofuran (THF), ethanol, ammonium hydroxide (NH4OH, 25–28%, aq.), hydrochloric acid (HCl, 36–38%, aq.), potassium dichromate (K2Cr2O7, recrystallized), sulfuric acid (H2SO4, 95–98%, aq.), nitric acid (HNO3, 65–68%, aq.) and phosphoric acid (H3PO4, ≥85%, aq.) were purchased from Beijing Chemical Works (Beijing, China). Azodiisobutyronitrile (AIBN), polyvinyl pyrrolidone (PVP, Mr = 104) and diphenylcarbazide were purchased from Fuchen Chemical Reagent Factory (Tianjin, China). In this work, all the materials were of analytical grade and used as received unless otherwise indicated. Deionized (DI) water prepared in our lab was employed for all the experimental processes.
Synthesis of PPy–PS composite microspheres
Typically, a proper amount of PVP and Py were dispersed in DI water (170 g) by using a Branson digital sonifier for 5 min at 50% amplitude with a 30 s pause per minute. The solution of AIBN (0.15 g in 10 g St) was slowly dropped into the Py aqueous dispersion under continuous magnetic stirring. Then the above ultrasonic process was repeated. The resulting emulsion was transferred into a 500 mL of four-neck flask equipped with a reflux condenser, a mechanical stirrer, a peristaltic pump, and the N2 inlet and outlet. The solution of APS (equivalent mole of Py, in 30 g water) was dropwise added into the flask at room temperature under continuous stirring. Subsequently, the reaction mixture was heated to 75 °C in a thermostated water bath. The reaction was stopped after 7 h. The product was separated by centrifugation and rinsed by DI water. The circle was repeated for at least 5 times until the upper liquid in the centrifugal tube was colorless and transparent. The final products were obtained as black powders after drying under vacuum at 60 °C for 12 h.
Batch adsorption experiments
Recrystallized K2Cr2O7 was employed to prepare a Cr(VI) stock solution. 200 mg of dry PPy–PS composite microspheres were directly added into a beaker containing 100 mL of different concentrations of Cr(VI) solution. During this process, the solution was kept magnetically stirred. At time zero and at selected time interval thereafter, 5 mL of the sample was withdrawn and filtered for testing.
Characterization
The morphology of samples were characterized by scanning electron microscope (SEM, TESCAN VEGA3 SBH, Czechic). The energy dispersive X-ray spectroscopy (EDX) analysis was performed by using Hitachi S-4700 (Japan). The transmission electron microscope (TEM) images were taken on Hitachi-800 (Japan). Elemental composition and chemical oxidation state of the surface and near surface species of the nanocomposites were determined by using X-ray photoelectron spectroscopy (XPS) on a Thermo VG ESCALAB 250 spectrometer (East Grinstead, UK) with a monochromatic Al Kα X-ray source. Residual amount of Cr(VI) in the solution were measured by using a UV-vis Spectrophotometer (UV-3150, SHIMADZU, Japan). The working wavelength was 540 nm.15 The time-dependent amount of Cr(VI) adsorbed per unit mass of adsorbent qt (mg g−1) is given by:16| |
 | (1) |
where t is the absorption time, Ct is the bulk-phase Cr(VI) concentration (mg L−1) at t, and m are the adsorbent mass (g). The percentage of Cr(VI) removal (R%) and the equilibrium adsorption capacity (qe, mg g−1) can be respectively calculated by the following equations:17| |
 | (2) |
| |
 | (3) |
where C0 is the initial concentration of Cr(VI) in solution (mg L−1), Ce is the equilibrium concentration (mg L−1), and V is the volume of solution (L).
Results and discussion
Synthesis of PPy–PS composite microspheres
The morphology of PPy–PS composite microspheres were characterized by using SEM and TEM (Fig. 1). In Fig. 1(a), all the objects are of uniform morphology, which are not regularly geospheres but dry-plum-like microsphere with the largely shrinking surface and the diameter of 3 μm. In Fig. 1(b), the small particles with a diameter from 100 to 200 nm can be found, which are accumulated on the continuous intact surfaces of the large spheres, resulting in the rough surface morphology. The sharp contrast between the dark edge and the pale centre in Fig. 1(c) suggests that the composite microspheres should be hollow inside. The average thickness of the shell is about 150 nm and the ratio of the wall thickness to the sphere diameter is 1/14. Besides, the detailed structure of the shell is revealed, implying that the composite microspheres are formed by a number of small particles. Some of these small particles are also hollow inside as shown in the zoom-in part of Fig. 1(c).
 |
| | Fig. 1 The typical SEM images (a and b), TEM images of PPy–PS composite microspheres (c) and the residual after THF extraction (d). | |
In order to further identify the particle-packing structure of the composite hollow microspheres, we extracted the PS matrix from the microspheres by using THF, after that only the insoluble PPy phase was remained. The typical TEM image of the microspheres after extraction (Fig. 1(d)) shows only small particles in the diameter below 200 nm in the observation field, which should be attributed to PPy. Moreover, both the morphology and size of these particles are consistent with those embedded on the sphere wall in Fig. 1(c). All the results infer that the original hollow microspheres are built up by nano-structured PPy particles using PS as the binder. After extraction by THF, the residual weight of PPy was weighed and the loading content of PPy on the microspheres is calculated to be about 30 wt%.
The EDX results in Table 1 reveal that the nitrogen element content in PPy–PS composite microspheres is 19.47%. After THF treatment, the nitrogen content in the rest part is 19.78%. Both of these values are almost equal and close to that in pure PPy (21.5%), further indicating that the small particles at the surface of microspheres are PPy. The hierarchical hollow structure with nano-PPy exposed outside is beneficial to the Cr(VI) removal from aqueous solution, which will be further discussed in the following part in this work.
Table 1 EDX composition results on the PPy–PS composite microspheres before and after THF extraction (only C, N, O elements are counted in calculation)
| Sample |
Elemental content/wt% |
| C |
N |
O |
| Before the extraction |
62.04 |
19.47 |
18.49 |
| After the extraction |
69.54 |
19.78 |
10.68 |
Structural evolution of PPy–PS composite microspheres
Structural evolution of the PPy–PS microspheres with reaction time was studied using SEM, and the typical images are displayed in Fig. 2. The reaction time of 0 min is identified as the time at which the reaction temperature just reached 75 °C after the addition of APS. The tiny particles (Fig. 2(a)) were found at 0 min, which should be formed by PPy oligomers due to the polymerization of Py initiated by APS in aqueous phase. The propagating PPy oligomers aggregated into tiny colloidal particles tailored by PVP.18 Being a kinetically stable system, the further propagation of PPy caused the destabilization of these particles, whose deposition and coalescence would be anchored by the hydrophobic droplet surface of St. We suppose that PPy colloidal precursor formed with the incorporation of PVP should be amphiphilic to localize at the O/W interface and stabilize the oil droplet as Pickering emulsifiers. The supposition is verified by Fig. 2(b), where the prototype of the microspheres was observed at 30 min of reactions. The droplets encapsulated by PPy particles would be the primary reaction loci for the following radical polymerization of St. With the further prolonged reaction time, the shape of microspheres became more and more identified, indicating by the increased monomer conversion and wall thickness. But the average size of the microspheres was almost constant with the prolonged reaction time from 30 to 420 min (Fig. 2(b)–(f)). Py monomer could exist in both the oil droplet and water phase. However, the further propagation of PPy was limited at the interface by the following factors: (i) the selection of water-soluble initiator of APS; (ii) the pre-location of precursor particles; (iii) the improved viscosity due to the polymerization inside the droplet. Not only the migration, but the size enlargement of PPy particles by coalescence was restricted by the improved local viscosity. Thus, PPy particles were decorated on the outmost surface of the microspheres to form a plum-like morphology. Meanwhile, during the solidification of shell, the migration of Py monomer from St droplet to the interface, and the capillary force due to the accumulation of PPy particles both acted as the driven forces to the emigration of the monomer swelled PS from the centre to the shell and left a void inside. In the dispersion with a droplet/particle size dispersity, there should be the limited coalescence of small particles into large ones, which can be used to explain the binding of sub-micron hollow particles onto the shell of the micron-sized spheres together with 30–50 nm of solid ones.19 During the post-treatment, such as heating and solvent evaporation, the deformation of the composite thin shell is expected to cause the typical dry-plum-like surface morphology in this work.20 The possible mechanism as described above is sketched in Scheme 1.
 |
| | Fig. 2 The SEM images of the PPy–PS composite microspheres at the reaction time of 0 min (a), 30 min (b), 60 min (c), 120 min (d), 240 min (e) and 420 min (f). The reaction time was recorded when the temperature reached 75 °C. | |
 |
| | Scheme 1 Schematic illustration of the synthetic procedure for dry plum-like PPy–PS composite microspheres: (A) at room temperature, the propagating PPy oligomers aggregated into tiny colloidal particles, and the particles acted as Pickering emulsifiers to localize on the O/W interface. (B) At 75 °C, Py and St polymerized together, and propagated synergistically. (C) After 7 h of polymerization, PPy–PS composite hollow microspheres spheres were obtained. | |
Effect of synthesis parameters
The influence of the content of Py was studied, in which the amount of Py monomer was various (0.3, 0.5, and 1.0 g) and that of St monomer was constant (10 g). The morphologies of these synthesized particles are displayed in Fig. 3(a) and (b), and 2(f), respectively. In Fig. 3(a), only the irregular lumps could be found, as Py monomer content is at 0.3 g. With the increasing Py content up to 0.5 g in Fig. 3(b), the spherical particles were achieved. However, the size of them is relatively larger (around 200 μm) than that in Fig. 2(f) (1–3 μm) as Py content at 1.0 g. Moreover, the particles in Fig. 3(b) show a wider size distribution than that in Fig. 2(f). Therefore, serving as Pickering emulsifiers, the in situ formed PPy particles are essential for the formation of composite microspheres, and the size of microspheres is controlled by the Py content. The increase of Py monomer content leads to the decrease of the sphere size.
 |
| | Fig. 3 The SEM images of PPy–PS composite microspheres prepared by different weight ratios of Py to PS: (a) 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, (b) 5 100, (b) 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100. 100. | |
In this study, PVP is employed as a co-stabilizer to tailor the structure formation. The effect of PVP content on the microsphere morphology was also investigated. It is found that the morphological variation of the microspheres as a function of PVP content is similar to that of Py monomer content. When the PVP content was 0.3 g or less, the regular spheres cannot be obtained (Fig. 4(a)). When PVP content was increased to 0.5 g (Fig. 4(b)), the product size was quite large, although the reaction could stably proceed. Moreover, some white product of PS was isolated after the reaction, resulting in the lower yield. The target micron-sized spheres as shown in Fig. 2(f) could only be obtained when the PVP content was 1.0 g and more. The final yield can be higher than 80%.
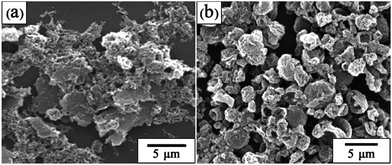 |
| | Fig. 4 The SEM images of PPy–PS composite microspheres prepared by different amount of PVP: (a) 0.3 g, (b) 0.5 g. | |
Reaction temperature also shows an obvious contribution to the microsphere structure. Taking into account the half-life of AIBN, the reaction temperature was varied from 65 to 80 °C in this work. When reaction temperature was 65 °C (Fig. 5(a)), only a small amount of shaped particles could be observed within a large amount of irregular ones, even though the reaction time was over 10 h. If the reaction temperature was risen to 70 °C (Fig. 5(b)), the individual particles with sphere-structure in micron size were observed. However, their shapes are still seriously deformed, which is similar to the case in Fig. 2(c). It is illustrated that both the low reaction temperature (Fig. 5(a) and (b)) and the insufficient reaction time (Fig. 2(c)) resulted to the low monomer conversion. So we suppose the deformation of the composites is not only controlled by the formation and distribution of PPy particles at the interfaces but also affected by the conversion of St monomer. Without the adequate participation of in situ generated PS, the PPy particles cannot be effectively bound, so that the highly controlled structure (same as that shown in Fig. 5(c)) would not be obtained. But when the reaction temperature was further increased to 80 °C, the massive substance in random state was obtained as shown in Fig. 5(d). The variation suggests that the synergism of the two polymerizations should be crucial on the morphology control. The high temperature would simultaneously accelerate the two polymerizations at a superfluous level. Instead of the controlled interfacial coalescence, therefore, the bulk coagulation would be dominated in this case.
 |
| | Fig. 5 The SEM images of PPy–PS composite microspheres prepared by different temperature: (a) 65 °C, (b) 70 °C, (c) 75 °C, (d) 80 °C. | |
Adsorption on Cr(VI) ions from water
As expected, the synthesized composite microspheres can adsorb the Cr(VI) ions from water. The appearance of the K2Cr2O7 solution before and after the addition of synthesized PPy–PS composite microspheres is displayed in Fig. 6. Before the adsorption by PPy–PS microspheres, the K2Cr2O7 solution was yellow and transparent (Fig. 6(a)). After the addition of PPy–PS at room temperature, the spheres were completely dispersed under gentle magnetic stirring as shown in Fig. 6(b). The adsorption on Cr(VI) ions was completed after 12 h. The microspheres spontaneously deposited down within 5 h. The upper solution was almost colorless in Fig. 6(c), demonstrating the applicability of composite microspheres as the Cr(VI) absorbents with an operating convenience.
 |
| | Fig. 6 A photographic representation of Cr(VI) removal from aqueous solution and precipitation separation of PPy–PS composite microspheres: (a) before adsorption, (b) during adsorption process, (c) after adsorption. | |
The adsorption of metal ions on the microspheres could be further revealed by XPS spectra. The wide-scan XPS spectrum of the microspheres before adsorption (Fig. 7(a)) shows three peaks at 284.7, 399.7, and 531.2 eV, corresponding to C 1s, N 1s and O 1s respectively. So there are C, N and O elements in the microspheres, which is consistent with the EDX result. After the adsorption, two additional peaks (577.1 and 587.5 eV respectively due to Cr 2p3/2 and Cr 2p1/2) appear in the wide-scan XPS spectrum (Fig. 7(b)), proving the existence of Cr element.21 It should be noted that the Cr 2p3/2 peak can be further divided into two valence state at 576.6 and 578.1 eV, which are in accordance with Cr(III) and Cr(VI), respectively. These results indicate the adsorption of Cr on the PPy–PS microspheres, and a part of the adsorbed Cr(VI) was reduced to Cr(III) by the electron rich PPy.22
 |
| | Fig. 7 XPS spectra of PPy–PS composite microspheres: (a) wide scan (b) Cr 2p. | |
Adsorption kinetics
The adsorption of PPy–PS composite microspheres on Cr(VI) was monitored, and R% is plotted as a function adsorption time in Fig. 7(a). It is clearly shown that the Cr(VI) ions can be rapidly adsorbed and about 80% of equilibrium adsorption capacity was achieved within 5 min when C0 was 10 mg L−1. The rapid adsorption rate at the initial period implies again that there should be much of the active PPy particles on the surface of the microspheres. However, the deep embedding of the active sites could not be excluded, which is expected to cause the depressed adsorption rate after 5 min. With the increasing Cr(VI) concentration (C0 = 10, 50, and 100 mg L−1), the prolonged final equilibrium time is obtained at 120, 180, and 420 min, respectively. The equilibrium adsorption capacity is about 50 mg g−1, as C0 is 100 mg L−1. When the initial Cr(VI) concentration is decreased to 50 or 10 mg L−1, the R% is improved to 83.5% and 100%, respectively.
The adsorption kinetics can be conducted on the basis of pseudo-first-order kinetic model (eqn (4)) and the pseudo-second-order kinetic model (eqn (5)):23
| |
ln(qe − qt) = k1t + ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe qe
| (4) |
| |
 | (5) |
where
k1 (min
−1) is the pseudo-first-order rate constant,
k2 (g mg
−1 min
−1) are the pseudo second-order rate constant, and
t is the adsorption time. The plots of ln(
qe −
qt) and
t/
qt as a function of
t are displayed in
Fig. 8(b) and (c), respectively. The parameters (
k1 and
k2) as well as the correlation coefficient (
R2) in the linear fitting are listed in
Table 2. Moreover, the calculated equilibrium adsorption capacity (
qe,cal: based on
eqn (4) and
(5)) and measured equilibrium adsorption capacity (
qe,exp: based on adsorption experiment) are also listed in
Table 2. The adsorption isotherms fits better to the pseudo-second-order model than to the pseudo-first-order model, suggesting that there should be not only a physical adsorption mechanism but also a chemical adsorption mechanism during the removal process of Cr(
VI) by the PPy–PS microspheres.
24 The
qe,cal is 29.73 mg g
−1 as the Cr(
VI) concentration at 100 mg L
−1, and decreases with the decreasing
C0.
 |
| | Fig. 8 (a) Sorption isotherms of Cr(VI) adsorption onto PPy–PS composite microspheres, (b) pseudo-first-order kinetic model for adsorption of Cr(VI) on the PPy–PS composite microspheres, (c) pseudo-second-order kinetic model for adsorption of Cr(VI) on the PPy–PS composite microspheres. | |
Table 2 Kinetically parameters for Cr(VI) adsorption onto PPy–PS composite microspheres
| C0 (mg L−1) |
qe,exp (mg g−1) |
Pseudo-first-order model |
Pseudo-second-order model |
| k1 × 103 (min−1) |
qe,cal (mg g−1) |
R2 |
k2 × 104 (g mg−1 min−1) |
qe,cal (mg g−1) |
R2 |
| 100 |
50 |
6.78 |
13.15 |
0.939 |
27.38 |
29.37 |
0.999 |
| 50 |
25 |
18.98 |
9.86 |
0.934 |
54.08 |
21.60 |
0.999 |
| 10 |
5 |
40.67 |
2.14 |
0.937 |
686.91 |
5.05 |
0.999 |
Adsorption isotherm
Both Langmuir isotherm model and Freundlich isotherm model are employed to describe the interaction between the adsorbate and adsorbent. Langmuir isotherm model is given by the following equation (eqn (6)).25 RL is the dimensionless separation factor, which is an essential characteristic of the Langmuir model for defining the favorability of an adsorption process, which is given by eqn (7):26| |
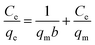 | (6) |
| |
 | (7) |
where qm (mg g−1) is the maximum adsorption capacity, and b is the Langmuir constant related to the energy of adsorption.
The Freundlich isotherm model is given by
| |
 | (8) |
where
Kf (mg g
−1) and 1/
n constants are related to the adsorption capacity and intensity of adsorption, respectively. The plots of
Ce/
qe as a function of
Ce are shown in
Fig. 9(a) and that of ln
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe
qe as a function of ln
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce
Ce is shown in
Fig. 9(b). The linear fitting results are listed in
Table 3, including the correlation coefficient (
R2). The higher value of
R2 in Langmuir model than that in Freundlich model reveals that Langmuir model is a better candidate for the description of the isotherm data. Suggested by the assumption of adsorption homogeneity of the model, the microspheres should represent the equally available adsorption sites, monolayer surface coverage, and no interaction between adsorbed species.
27 Meanwhile, the result of
RL = 0.032 is in the range from 0 to 1, indicating that the adsorption process is favorable. The maximum adsorption quality (
qm) is 46.8 mg g
−1, which is much higher than that of the PPy/wood sawdust composite (3.4 mg g
−1)
4 and activated carbon (15.47 mg g
−1).
28 Compared with the pure PPy nanoparticles (
qm = 180 mg g
−1),
29 maximum adsorption quality of the synthesized PPy–PS microspheres is 27% of that value. However, the PS used in the matrix is inert in adsorption. Based on the loading amount of PPy on the microspheres (30 wt%), the
qm,PPy should be 157 mg g
−1, which is very similar to that of pure PPy nanoparticles and slightly higher than that of Fe
3O
4/PPy composite microspheres (101.04 mg g
−1).
30 It can be seen that the adsorption capability of nano-structured PPy is largely sustained in our micron-sized composite hollow spheres prepared
via PMHP method. Meanwhile, due to the micron size, the synthesized PPy–PS spheres are easy to be dispersed and collected in practices.
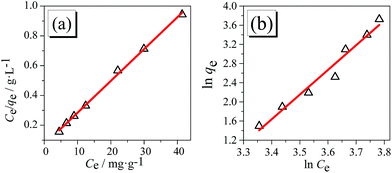 |
| | Fig. 9 Adsorption isotherms data fitted to (a) Langmuir model, (b) Freundlich model. | |
Table 3 Langmuir and Freundlich isotherm parameters of the Cr(VI) adsorption on PPy–PS microspheres
| Langmuir constants |
Freundlich constants |
| qm (mg g−1) |
b (L mg−1) |
RL |
R2 |
Kf (mg g−1) |
1/n |
R2 |
| 46.8 |
0.302 |
0.032 |
0.998 |
22.17 |
0.188 |
0.962 |
Conclusions
We demonstrate the preparation of PPy–PS composite hollow microspheres via a feasible PMHP synthesis procedure, which was carried out in a multicomponent heterogeneous condition by properly dispersing all the monomers and part of the initiators in water. The oxidation polymerization of Py and the radical polymerization of St were initiated in sequence by controlling on the initiator addition and temperature elevation protocol. The synergism of the two polymerizations resulted in the formation of composite microspheres with the diameter of about 3 μm. The SEM, TEM, and EDX results reveal the hollow and dry-plum-like morphology of the synthesized composite microspheres, whose wall is built by the nano-structured PPy particles bound by PS. The interfacial location of the in situ generated PPy particles should be essential for the formation of the hollow spheres, which was influenced by the relative Py content, the dispersant PVP content, and the reaction temperatures. The loading of PPy particles on the hollow spheres is also approved by their capability to remove Cr(VI) from water. The decoration of PPy particles at the surface of microspheres leads to the effective removal of Cr(VI) from water. Moreover, the kinetic results indicate that the adsorption process can be well fitted by the pseudo-second order model. Langmuir isotherm model is applicable to describe the equilibrium isotherm data. Considering on the feasible synthesis and hierarchical hollow structure, the synthesized PPy–PS composites microspheres are of potential applications as adsorbent to remove Cr(VI) from industrial waste water.
Acknowledgements
The authors are grateful for the financial support from the National Natural Science Foundation of China (Grant No. 21304007), and the Fundamental Research Funds for the Central Universities (JD1510 and buctrc 201614).
Notes and references
- S. A. Katz and H. Salem, J. Appl. Toxicol., 1993, 13, 217 CrossRef CAS PubMed; M. A. Behnajady and S. Bimeghdar, Chem. Eng. J., 2014, 239, 105 CrossRef.
- N. Adhoum, L. Monser, N. Bellakhal and J. E. Belgaied, J. Hazard. Mater., 2004, 112, 207 CrossRef CAS PubMed; C. C. C. Wang, Y. Chang and C. Y. Chen, Macromol. Chem. Phys., 2001, 202, 882 CrossRef; E. Salehi and S. S. Madaeni, Appl. Surf. Sci., 2014, 288, 537 CrossRef; W. J. Yang, P. Ding, L. Zhou, J. G. Yu, X. Q. Chen and F. P. Jiao, Appl. Surf. Sci., 2013, 282, 38 CrossRef; S. He, Y. F. Zhao, M. Wei and X. Duan, Ind. Eng. Chem. Res., 2011, 50, 2800 CrossRef; M. C. Liu, W. Tao, X. L. Wu, C. L. Chen, J. Hu, J. Li and X. K. Wang, Dalton Trans., 2013, 14710 RSC; Y. Yuan, G. H. Zhang, Y. Li, G. L. Zhang, F. B. Zhang and X. B. Fan, Polym. Chem., 2013, 4, 2164 RSC; H. Wang, X. Yuan, Y. Wu, X. Chen, L. Leng, H. Wang, H. Li and G. Zeng, Chem. Eng. J., 2015, 262, 597 CrossRef.
- M. Hunsom, K. Pruksathorn, S. Damronglerd, H. Vergnes and P. Duverneuil, Water Res., 2005, 39, 610 CrossRef CAS PubMed.
- R. Ansari and N. K. Fahim, React. Funct. Polym., 2007, 67, 367 CrossRef CAS.
- C. Yao, Y. Xu, Y. Kong, W. Liu, W. Wang, Z. Wang and Y. Wang, Appl. Clay Sci., 2012, 67–68, 32 CrossRef CAS.
- K. Z. Setshedi, M. Bhaumik, M. S. Onyango and A. Maity, Chem. Eng. J., 2015, 262, 921 CrossRef CAS.
- J. Ormond-Prout, D. Dupin and S. Armes, J. Mater. Chem., 2009, 19, 1433 RSC; M. Redondo, M. Garcia, E. S. D. L. Blanca, M. Pablos, I. Carrillo, M. Gonzalez-Tejera and E. Enciso, Polymer, 2010, 51, 1728 CrossRef CAS; X. Shi, A. L. Briseno, R. J. Sanedrin and F. Zhou, Macromolecules, 2003, 36, 4093 CrossRef; X. Yang, T. Dai, M. Wei and Y. Lu, Polymer, 2006, 47, 4596 CrossRef.
- X. Lu, H. Mao and W. Zhang, Polym. Compos., 2009, 30, 847 CrossRef CAS.
- O. Kreye, T. Toth and M. A. R. Meier, J. Am. Chem. Soc., 2011, 133, 1790 CrossRef CAS PubMed; A. Sehlinger, R. Schneider and M. A. R. Meier, Eur. Polym. J., 2014, 50, 150 CrossRef; X. X. Deng, L. Li, Z. L. Li, A. Lv, F. S. Du and Z. C. Li, ACS Macro Lett., 2012, 1, 1300 CrossRef; Y. Z. Wang, X. X. Deng, L. Li, Z. L. Li, F. S. Du and Z. C. Li, Polym. Chem., 2013, 4, 444 RSC.
- A. Chen, H. Wang and X. Li, Chem. Commun., 2005, 14, 1863–1864 RSC.
- J. Zhang, T. Qiu, H. Yuan, W. Shi and X. Li, Mater. Lett., 2011, 65, 790–792 CrossRef CAS.
- J. Zhang, T. Qiu, S. Ren, H. Yuan, L. He and X. Li, Mater. Chem. Phys., 2012, 134, 1072 CrossRef CAS.
- H. Yang, L. Fu, L. Wei, J. Liang and B. P. Binks, J. Am. Chem. Soc., 2015, 137, 1362 CrossRef CAS PubMed; S. B. Haaj, W. Thielemans, A. Magnin and S. Boufi, ACS Appl. Mater. Interfaces, 2014, 6, 8263 Search PubMed; S. Yang, C. Song, T. Qiu, L. Guo and X. Li, Langmuir, 2013, 29, 92 CrossRef PubMed.
- C. H. Wu, W. Y. Chiu and T. M. Don, Polymer, 2012, 53, 1086 CrossRef CAS.
- APHA, AWWA and WPCF, in Standard methods for examination of water and wastewater, American public health association, Washington, DC, 14th edn, 1975, p. 192 Search PubMed.
- M. Costa, Toxicol. Appl. Pharmacol., 2003, 188, 1 CrossRef CAS PubMed.
- IARC (International Agency for Research on Cancer), IARC monographs on the evaluation of carcinogenic risks to humans: overall evaluation of carcinogenicity, in: An updating of IARC monographs, Suppl. 7, WHO, Lyon, France, 1987, vol. 1–42 Search PubMed; S. A. Katz and H. T. Salem, Environ. Pollut., 1994, 89, 117 Search PubMed.
- C. Nicolas, M. O. Timothy and V. Brian, J. Chem. Soc., Chem. Commun., 1988, 17, 1189 Search PubMed.
- S. Tesch, C. Gerhards and H. Schubert, J. Food Eng., 2002, 54, 167 CrossRef.
- T. Chen, P. J. Colver and S. A. F. Bon, Adv. Mater., 2007, 19, 2286 CrossRef CAS.
- O. A. Kuznetsov, O. N. Sorokina, V. G. Leontiev, O. A. Shlyakhtin, A. L. Kovarski and A. A. Kuznetsov, J. Magn. Magn. Mater., 2007, 311, 204 CrossRef CAS.
- J. Wang, C. Luo, G. Qi, K. Pan and B. Cao, Appl. Surf. Sci., 2014, 316, 245 CrossRef CAS; M. Bhaumik, A. Maity, V. V. Srinivasu and M. S. Onyango, J. Hazard. Mater., 2011, 190, 381 CrossRef PubMed.
- Y. S. Ho and G. McKay, Process Biochem., 1999, 34, 451 CrossRef CAS.
- J. Hu, G. H. Chen and I. M. C. Lo, Water Res., 2005, 39, 4528 CrossRef CAS PubMed.
- H. Wang, X. Yuan, G. Zeng, L. Leng, X. Peng, K. Liao, L. Peng and Z. Xiao, Environ. Sci. Pollut. Res., 2014, 21, 11552 CrossRef CAS PubMed.
- K. R. Hall, L. C. Eagletow, A. Acrivers and T. Vermeulen, Ind. Eng. Chem. Res., 1966, 5, 212 CAS.
- S. P. Rigby, Langmuir, 2002, 18, 1613 CrossRef CAS.
- B. Sandhya and A. K. Tonni, Chemosphere, 2004, 54, 951 CrossRef PubMed.
- T. Yao, T. Cui, J. Wu, Q. Chen, S. Lu and K. Sun, Polym. Chem., 2011, 2, 2893 RSC.
- Y. Wang, B. Zou, T. Gao, X. Wu, S. Lou and S. Zhou, J. Mater. Chem., 2012, 22, 9034 RSC.
Footnote |
| † Author contributions: Fengdan Hu and Longhai Guo contributed equally to this work. |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. 




![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, (b) 5
100, (b) 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100.
100.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe
qe

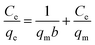


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe as a function of ln
qe as a function of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce is shown in Fig. 9(b). The linear fitting results are listed in Table 3, including the correlation coefficient (R2). The higher value of R2 in Langmuir model than that in Freundlich model reveals that Langmuir model is a better candidate for the description of the isotherm data. Suggested by the assumption of adsorption homogeneity of the model, the microspheres should represent the equally available adsorption sites, monolayer surface coverage, and no interaction between adsorbed species.27 Meanwhile, the result of RL = 0.032 is in the range from 0 to 1, indicating that the adsorption process is favorable. The maximum adsorption quality (qm) is 46.8 mg g−1, which is much higher than that of the PPy/wood sawdust composite (3.4 mg g−1)4 and activated carbon (15.47 mg g−1).28 Compared with the pure PPy nanoparticles (qm = 180 mg g−1),29 maximum adsorption quality of the synthesized PPy–PS microspheres is 27% of that value. However, the PS used in the matrix is inert in adsorption. Based on the loading amount of PPy on the microspheres (30 wt%), the qm,PPy should be 157 mg g−1, which is very similar to that of pure PPy nanoparticles and slightly higher than that of Fe3O4/PPy composite microspheres (101.04 mg g−1).30 It can be seen that the adsorption capability of nano-structured PPy is largely sustained in our micron-sized composite hollow spheres prepared via PMHP method. Meanwhile, due to the micron size, the synthesized PPy–PS spheres are easy to be dispersed and collected in practices.
Ce is shown in Fig. 9(b). The linear fitting results are listed in Table 3, including the correlation coefficient (R2). The higher value of R2 in Langmuir model than that in Freundlich model reveals that Langmuir model is a better candidate for the description of the isotherm data. Suggested by the assumption of adsorption homogeneity of the model, the microspheres should represent the equally available adsorption sites, monolayer surface coverage, and no interaction between adsorbed species.27 Meanwhile, the result of RL = 0.032 is in the range from 0 to 1, indicating that the adsorption process is favorable. The maximum adsorption quality (qm) is 46.8 mg g−1, which is much higher than that of the PPy/wood sawdust composite (3.4 mg g−1)4 and activated carbon (15.47 mg g−1).28 Compared with the pure PPy nanoparticles (qm = 180 mg g−1),29 maximum adsorption quality of the synthesized PPy–PS microspheres is 27% of that value. However, the PS used in the matrix is inert in adsorption. Based on the loading amount of PPy on the microspheres (30 wt%), the qm,PPy should be 157 mg g−1, which is very similar to that of pure PPy nanoparticles and slightly higher than that of Fe3O4/PPy composite microspheres (101.04 mg g−1).30 It can be seen that the adsorption capability of nano-structured PPy is largely sustained in our micron-sized composite hollow spheres prepared via PMHP method. Meanwhile, due to the micron size, the synthesized PPy–PS spheres are easy to be dispersed and collected in practices.






