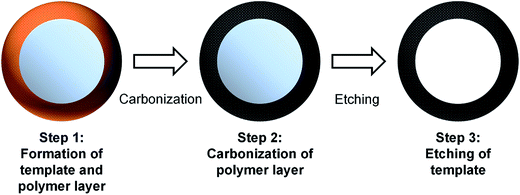
Porous carbon hollow spheres synthesized via a modified Stöber method for capacitive deionization†
Zhi Yi Leongab and
Hui Ying Yang*a
aPillar of Engineering Product Development (EPD), Singapore University of Technology and Design, 8 Somapah Road, Singapore 487372. E-mail: yanghuiying@sutd.edu.sg
bHyflux Innovation Centre, 80 Bendemeer Rd, Singapore 339949
First published on 18th May 2016
Abstract
Capacitive deionization (CDI) is a promising desalination technology that is environmentally friendly and requires far less energy when compared with conventional technologies such as reverse osmosis. One of the main strategies used to improve the desalination performance is to synthesize materials that are electrically conductive, have high surface areas and are chemically stable. In this work, porous carbon hollow spheres of various sizes were synthesized using a modified Stöber process. The morphology, porosity and electrochemical properties of the spheres are studied using SEM, TEM, BET and CV techniques while their desalination performances are evaluated based on batch-mode CDI experiments. Based on our results, it is found that spheres of smaller sizes exhibit larger specific surface areas, higher specific capacitances and electrosorption capacities. The largest surface area of 809.91 m2 g−1 was obtained for a carbon hollow sphere of approximately 230 nm and a salt removal capacity of up to 18.88 mg g−1 was observed. Besides that, the electrosorption isotherms and kinetics were studied and reported.
1. Introduction
The lack of access to clean and portable water supplies is a debilitating issue currently affecting many countries, especially those which are underdeveloped or developing. According to several water research reports, this problem is further exacerbated by an exponential growth in human population, urban renewal projects, drastic climate changes and man-made pollution.1,2 Technological advancement in water treatment strategies has made large scale seawater desalination a reality and while this has mitigated some of the water woes, it is far from a sustainable solution. Traditional desalination processes involve either thermal distillation or membrane filtration which require costly membranes, high-pressure pumps, thermal heaters or distillation columns.3,4 Thus, traditional desalination remains one of the most expensive and energy intensive solutions available and it is imperative that we develop alternative solutions for a sustainable water future.A potentially low cost and less energy intensive solution would be capacitive deionization (CDI). CDI can be thought of as a derivative of the electrical double layer (EDL) supercapacitor since the two systems share a similar mechanism of charge sequestering on the electrode–electrolyte interface.5 Briefly put, CDI refers to the removal of charged ionic species by means of electrostatic adsorption when an electric field is imposed across two porous electrodes. A typical CDI cell consists of two porous electrodes (usually carbonaceous materials) of approximately 100–500 μm (ref. 6) and a spacer channel of about 200 μm between them. During an operation of a CDI cell, saline water is first passed through the spacer channel while a direct current (DC) potential or current is applied to the electrodes. An electric field is created between the electrodes and cations from the water are attracted to the negatively charged electrode whereas anions are attracted to the positively charged electrode. The ions are captured within electrical double layers formed inside the intraparticle pores and when these pores are saturated with ions, the electrodes is said to have reached maximum adsorption capacity. To regenerate the electrodes, a short circuit is usually applied. Once the electric field is removed, the ions return to the water and a concentrated effluent stream is produced. Since the mechanism of CDI is a purely physisorption process, continuous adsorption and regeneration will have little impact on the material and it remains usable over many cycles.
Since the operational voltage windows for CDI is less than the electrolysis voltage of water (∼1.23 V),6 the energy requirements of CDI can be significantly lower than conventional desalination systems.5,7 Leveraging on this advantage, researchers have also developed solar powered CDI modules that can provide access to portable water in remote areas.8 Furthermore, the CDI process does not necessitate the use of membranes or high osmotic pressures which translates to a potentially scalable technology.7 Lastly, electrode materials used for CDI are usually environmentally benign and the adsorption/desorption processes do not release any pollutants or contaminants. Hence, CDI is a green technology that is suitable for our water needs. Despite the promising outlook, CDI still remains a relatively new field of science and trails behind the more mature technologies such as reverse osmosis or multi-stage flash distillation.
In recent years, there has been a considerable amount of research efforts dedicated to the development of novel materials for enhanced electrosorption capacity, specifically carbon materials.3,9–15 Carbon materials are chemically inert, can be easily functionalized with various precursors and have many nanostructured derivatives that possess high specific surface areas (SSAs) and high electrical conductivities.9 Carbon materials typically used in CDI include activated carbon,16–18 carbon nanotubes,19 carbon aerogels,20 graphene composites21,22 and mesoporous carbons.23–25 Current state of art is focused on the synthesis of new materials and composites that possess conductive and adsorptive properties that are superior to their constituents. For example, polypyrrole and MnO2 were added to graphene oxide to produce a 3D hierarchical structure with a high specific surface area and a porous network.26 The polypyrrole functions as a conductive scaffolding to prevent restacking of graphene oxide sheets and to boost electrical conductivity across the composite.26 On the other hand, MnO2 was added to enhance the specific capacitance. The graphene–polypyrrole–Mn composite managed to achieve a specific electrosorptive capacity of 18.4 mg g−1 and a specific capacitance of 356 F g−1.26 Although composites have been shown to improve CDI,9 other efforts have been directed to the synthesis and application of new carbon materials. One area of focus that has seen success in electrochemical storage applications is porous carbon spheres.
Porous carbon spheres and spherical colloids have regular geometries and high specific surface areas that can be easily functionalized with precursors. Furthermore, the pore size distribution and type of porosity can be managed by various synthesis conditions through a rational design process.27 In lithium battery research, Chen et al. used micro-sized porous carbon spheres to achieve a reversible capacity of 150 mA h g−1 at a discharge current of 20 A g−1.28 And in supercapacitor research, Ferrero et al. employed N-doped porous carbon capsules in an aqueous electrolyte to produce a specific capacitance of 240 F g−1. Despite this, the use of carbon spheres in CDI research is relatively new and unexplored. The more recent work involved N-doped porous carbon spheres29 and graphene-coated hollow mesoporous carbon spheres.30 Although both pieces of work document the electrosorption capabilities of carbon spheres, they do not explain how changes in the spherical structure affect the CDI performance.
In this work, we investigate the synthesis and characterization of carbon hollow spheres (CHS) of various sizes using a modified Stöber method and their applications in CDI. The Stöber method is a well-known method used to prepare silica spheres31 and has recently been extended to synthesize resorcinol formaldehyde (RF) polymeric spheres.32,33 This method is relatively straightforward and is well-suited for preparing spheres of various sizes. We had prepared spheres of 3 different sizes and investigated the effects of size on the Brunauer, Emmett and Teller (BET) surface area (m2 g−1), the pore size distribution and specific capacitance (F g−1). The CDI performance is studied in electrosorption experiments under different experimental conditions and evaluated based on established metrics such as electrosorption capacity (mg g−1) and charge efficiency.7
2. Experimental section
2.1 Synthesis of carbon hollow spheres (CHS)
Carbon hollow spheres of various sizes were prepared according to prior literature.33 In general, the synthesis of carbon hollow spheres follows a three step process that is illustrated in Fig. 1. The first step describes the formation of a RF polymer layer over a silica core which is subsequently carbonized in the second step. The concurrent formation of the RF layer and silica core is possible due to the different rate of reactions for both processes.33 The formation of SiO2 particles occur almost immediately with the addition of TEOS. The formation of monodisperse SiO2 spheres is also facilitated by the adhesion of ammonium ions (NH4+) on the surface of the particles. This stabilizes the colloid suspension and prevents aggregation of the SiO2 particles.32–34 On addition of resorcinol and formaldehyde, hydroxymethyl substituted species are formed and these species diffuse towards the SiO2 particles due to electrostatic attraction. Condensation between these species result in the formation of a polymeric layer and further cross-linking of these species occur via hydrothermal treatment.32–34 The final step is to etch away the silica core to create a hollow carbon sphere. The size of the sphere is varied by changing the amount of tetraethyl orthosilicate (TEOS, purity > 98%) added.In a typical synthesis, 1.25 mL of aqueous ammonia (28.0–30.0 wt%) was added dropwise into a 32 mL mixture of ethanol and deionized water (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) and stirred for 30 min at 30 °C in an oil bath. Next, a certain amount of TEOS (1 mL, 3 mL and 5 mL) with 0.2 g of resorcinol and 0.28 mL of formaldehyde solution (35 wt%) was added to the mixture. The resulting mixture was stirred at 30 °C for 12 h before it was transferred to a Teflon lined autoclave to be sealed and heated at 100 °C for 24 h. RF polymer coated spheres were obtained at the end of the hydrothermal step and were collected by centrifugation at 8000 rpm for 15 min. The spheres were washed with ethanol and deionized water before drying in an oven at 60 °C overnight. The dried powder was then annealed at 800 °C for 1 h under a N2 atmosphere at a heating rate of 5 °C min−1 in a tubular furnace. Finally, the carbonized products were etched with HF for 12 h and washed several times with ethanol and deionized water until pH was returned to neutral. The carbon spheres obtained were designated CHS 1, CHS 3 and CHS 5 according to the amount of TEOS added.
1, v/v) and stirred for 30 min at 30 °C in an oil bath. Next, a certain amount of TEOS (1 mL, 3 mL and 5 mL) with 0.2 g of resorcinol and 0.28 mL of formaldehyde solution (35 wt%) was added to the mixture. The resulting mixture was stirred at 30 °C for 12 h before it was transferred to a Teflon lined autoclave to be sealed and heated at 100 °C for 24 h. RF polymer coated spheres were obtained at the end of the hydrothermal step and were collected by centrifugation at 8000 rpm for 15 min. The spheres were washed with ethanol and deionized water before drying in an oven at 60 °C overnight. The dried powder was then annealed at 800 °C for 1 h under a N2 atmosphere at a heating rate of 5 °C min−1 in a tubular furnace. Finally, the carbonized products were etched with HF for 12 h and washed several times with ethanol and deionized water until pH was returned to neutral. The carbon spheres obtained were designated CHS 1, CHS 3 and CHS 5 according to the amount of TEOS added.
All chemicals with the exception of hydrofluoric acid (HF) used in the synthesis and preparation of the electrodes were purchased from Sigma Aldrich. HF was purchased from Best Chemical and diluted to 10% before use. All other chemicals were used without further purification. Deionized water for synthesis was obtained from an ultrapure water purification system (Sartorius, Arium Pro).
2.2 Characterization
The structure and morphology of the as-prepared carbon hollow spheres were studied using field emission scanning electron microscopy (FE-SEM, JEOL JSM-7600F) and transmission electron microscopy (TEM, JEM-2010). Before morphological studies were performed, the powdered samples were first dispersed in ethanol by ultrasonication for 10 min and allowed to dry on the sample holder. The degree of graphitization and structural defects were also investigated by Raman spectroscopy using a WITec alpha 300 confocal Raman microscope equipped with a 532 nm solid-state laser for excitation. The BET surface area and pore size distribution were investigated by measuring the nitrogen sorption isotherms at 77 K using an Autosorb-iQ-MP-XR system (Quantachrome). Before nitrogen sorption experiments were performed, the sample was degassed at 200 °C for 6 h to remove any moisture or contaminants. Pore size distribution was determined from the desorption branches using the Barrett–Joyner–Halenda (BJH) model. Electrochemical measurements were carried out using a 3-electrode setup with the working electrode composed of CHS (1, 3 or 5), platinum foil as the counter electrode and saturated calomel electrode (SCE) as the reference. Both the working and counter electrode have an effective surface area of 1.5 × 1.5 cm2. Cyclic voltammetry (CV) measurements were performed in solutions of various concentrations (0.05 M, 0.5 M and 1 M) and under different scan rates (0.5 mV s−1, 1 mV s−1, 5 mV s−1 and 10 mV s−1). All electrochemical measurements were performed using an electrochemical workstation (VMP3, Bio-logic).2.3 Electrosorption experiments
The desalination performance of CHS electrodes was evaluated by performing batch-mode CDI experiments using a continuously recycling system as described in a previous work.17 CDI electrodes were first fabricated by preparing a mixture of CHS, acetylene black, polyvinylidene fluoride (PVDF) in a mass ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with 3 mL of N-methyl-2-pyrrolidone (NMP). The mixture was thoroughly mixed into a uniform slurry and cast onto graphite sheets of 5 × 5 cm2 using a doctor blade. The coating thickness was approximately 80 μm and mass loading was about 60 mg. Finally, the electrodes were dried in an oven at 60 °C overnight before use. The solutions used for the experiments were analytical NaCl solutions of 250 to 750 mg L−1 and the applied voltage was varied from 1.0 to 1.6 V using a potentiostat (SMU 2450, Keithley). Other experimental parameters which remained constant were the flow rate (30 mL min−1), solution temperature (298 K) and total volume of solution (50 mL). The relationship between solution conductivity and salt concentration was obtained according to a calibration table made prior to the experiment. The conductivity meter (DDSJ-308F, Leici) was positioned near the outlet of the CDI cell and a proprietary data logging software was used to measure the conductivity of the solution every 10 s. The salt electrosorption capacity, Γ (mg g−1) is calculated as:
1 with 3 mL of N-methyl-2-pyrrolidone (NMP). The mixture was thoroughly mixed into a uniform slurry and cast onto graphite sheets of 5 × 5 cm2 using a doctor blade. The coating thickness was approximately 80 μm and mass loading was about 60 mg. Finally, the electrodes were dried in an oven at 60 °C overnight before use. The solutions used for the experiments were analytical NaCl solutions of 250 to 750 mg L−1 and the applied voltage was varied from 1.0 to 1.6 V using a potentiostat (SMU 2450, Keithley). Other experimental parameters which remained constant were the flow rate (30 mL min−1), solution temperature (298 K) and total volume of solution (50 mL). The relationship between solution conductivity and salt concentration was obtained according to a calibration table made prior to the experiment. The conductivity meter (DDSJ-308F, Leici) was positioned near the outlet of the CDI cell and a proprietary data logging software was used to measure the conductivity of the solution every 10 s. The salt electrosorption capacity, Γ (mg g−1) is calculated as:
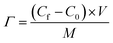 | (1) |
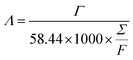 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485 (C mol−1).
485 (C mol−1).
3. Results and discussion
3.1 Morphological characteristics
The spherical structure of the carbon hollow spheres can be seen in SEM and TEM images shown in Fig. 2. CHS 1 and 3 both show smooth textures whereas a rougher surface is observed for CHS 5. The mean particle size of carbon hollow spheres is determined by measuring the diameters of uniform spheres using an image processing software (ImageJ) and the statistical distributions are shown in the inserts of Fig. 2. Briefly, the diameter of CHS 1 is 230 ± 20.90 nm, CHS 3 is 286 ± 17.85 and CHS 5 is 503 ± 66.08. The increase in diameter is attributed to the increase in size of the silica template by adding larger amounts of TEOS. Other ways of manipulating the sphere size include changing the ethanol to water ratio and/or the amount of ammonia added.32,33 A smaller sphere size is desirable for CDI since it implies a larger surface area for electrosorption of ions. The TEM images show a hollow structure for all CHS samples and the thickness of the carbon layer is estimated to be 12.9 ± 3.45 nm. HRTEM images are shown in Fig. S3(a) and (b)† and it can be observed that the surface of the carbon spheres are amorphous in nature. This is further confirmed by the selected area electron diffraction (SAED) pattern which clearly shows the amorphous rings.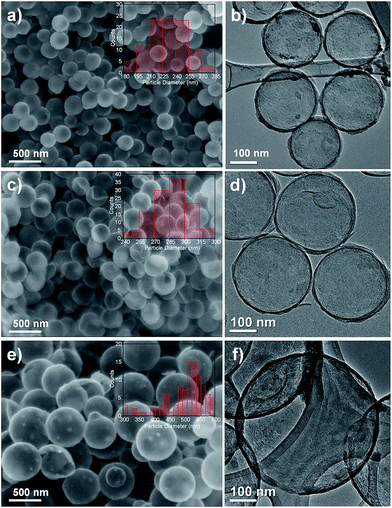 | ||
| Fig. 2 FESEM images of (a) CHS 1, (c) CHS 3 and (e) CHS 5 (inset shows the size distribution of the spheres). TEM images of (b) CHS 1, (d) CHS 3 and (f) CHS 5. | ||
Raman spectroscopy was performed to investigate the degree of graphitization and defects on the carbon sphere surface. From the Raman spectra in Fig. S1,† we observe two obvious graphitic peaks located at 1322 cm−1 (D band) and 1359 cm−1 (G band) for all CHS samples. The D band characterizes the structural disorder in the carbon matrix and the G band is attributed to the first-order scattering of E2g mode at the Γ-point.30,35 The intensity ratio of D to G band, Id/Ig is related to the degree of disorder where a lower value implies less disorder and consequently a higher degree of graphitization.30 The Id/Ig values are 1.00 for CHS 1, 1.021 for CHS 3 and 0.986 for CHS 5. The similar Id/Ig values indicate a similar carbon structure that is unaffected by size differences.
The nitrogen sorption isotherms were studied and a summary of the surface area, average pore size and total volume is recorded in Table 1. The spheres exhibit typical Type IV isotherms (Fig. 3(a)) which indicates an abundance of mesopores. This is expected since silica templates are known to impart mesoporous properties to the products formed from it.36 From the isotherms presented in Fig. 3(a), we observe both an increase in volume of N2 adsorbed and a widening of the hysteresis loop as the size of the sphere decreases. The largest SSA is observed for CHS 1 (809.91 m2 g−1) which has the smallest particle size. The pore size distribution is analysed using the BJH model and the results are shown in Fig. 3(b). Peaks in pore size distribution are observed for CHS 1 and 3 which indicates a more developed mesoporous structure as compared to that of CHS 5. CHS 1 in particular has two sharp peaks at about 4.18 nm and 6.54 nm. It is generally agreed that mesopores act as efficient transport pathways for ion diffusion and transport and this can result in high specific capacitances and ion adsorption capacities.10,23
| SBET (m2 g−1) | Pore size (nm) | Pore volume (cm3 g−1) | |
|---|---|---|---|
| CHS 1 | 809.91 | 4.18 | 0.673 |
| CHS 3 | 173.87 | 10.058 | 0.228 |
| CHS 5 | 136.62 | 3.933 | 0.221 |
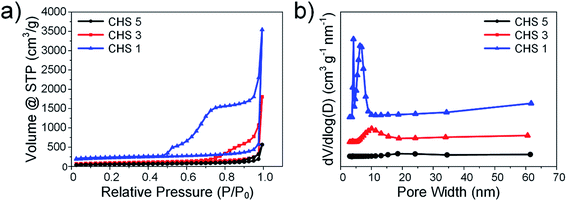 | ||
| Fig. 3 (a) Nitrogen sorption isotherms of CHS 1, CHS 3 and CHS 5. (b) BJH pore size distributions of CHS 1, CHS 3 and CHS 5. | ||
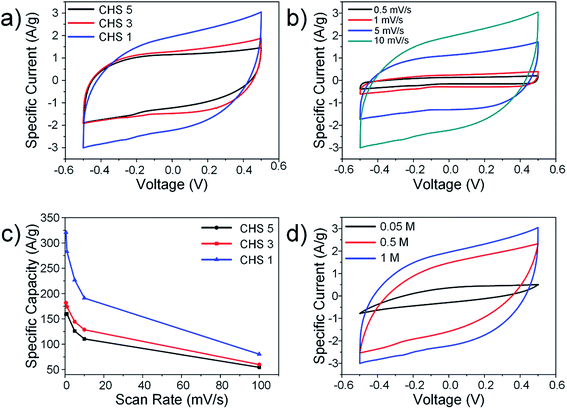
Since ion adsorption in CDI occurs via the formation of EDLs, methods used to characterize supercapacitors are also relevant for CDI.6,7 CV experiments were performed using a 3-electrode setup and the effects of sphere size, solution concentration and scan rate were evaluated. The representative CV curves for all CHS samples are shown in Fig. 4(a) and although the voltammogram of CHS 1 shows slight distortion, no obvious faradic peaks were observed. The general shape of the CV curves is rectangular and show typical EDL behaviour. The specific capacitances calculated from the voltammogram areas are 191.15 F g−1 for CHS 1, 128.58 F g−1 for CHS 3 and 110.52 F g−1 for CHS 5. The increase in specific capacitance is consistent with an increase in SSAs due to smaller sphere sizes. The CV curves were further investigated by varying the scan rate from 0.5 mV s−1 to 100 mV s−1 (Fig. 4(b)) in 1 M NaCl solution using CHS 1. For the sake of clarity, the graph at a scan rate of 100 mV s−1 is not shown. The graphs at various scan rates show a distinct rectangular shape and as the scan rates increase, distortions in the CV curve are observed due to increased resistance and slower ion transportation.37 There is insufficient time for ions to diffuse into pores and this results in a decay of capacitance at higher scan rates. The decrease in specific capacitance is also observed for both CHS 3 and 5 as shown in Fig. 4(c). The effect of solution concentration is studied and the results are shown in Fig. 4(d). The specific capacitance increases with increasing solution concentration and this is due to the compression of the EDL thickness at higher concentrations.38,39 The smaller EDL thickness results in less overlapping and hence, greater adsorption of ions.
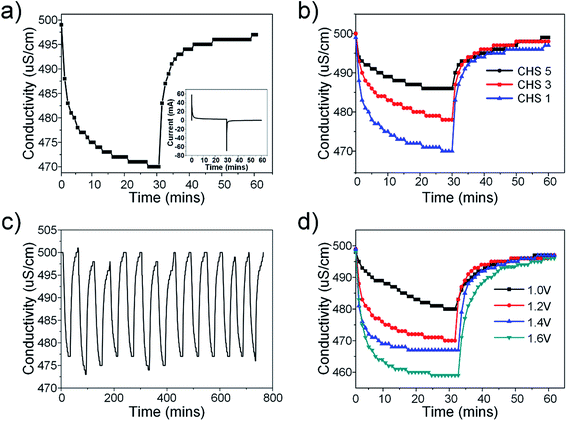
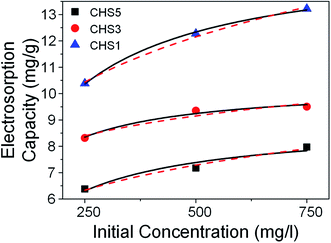
The desalination performance was evaluated based on batch-mode experiments performed using symmetric electrodes in a custom-made cell. The cell was first flushed with DI water before the experiments began. Stock NaCl solutions of various concentrations were prepared beforehand and the solution was allowed to pass through the cell until the conductivity of the effluent solution was constant. Electrosorption experiments were conducted by applying a voltage across the electrodes during ion adsorption and short circuiting during ion desorption. The electrosorption results of CHS 1 in 250 mg L−1 NaCl solution at 1.2 V is presented in Fig. 5(a). Equilibrium was reached when the conductivity of the effluent solution reached a minimum value. The transient current graph shown in the inset shows typical capacitive behaviour. The comparative results of CHS 1, 3 and 5 are shown in Fig. 5(b) and CHS 1 shows the highest salt removal capacity of 10.39 mg g−1. This result can be attributed to the high accessible surface areas of the mesoporous spheres. The adsorption–desorption curve of CHS 1 is shown in Fig. 5(c) and it shows a cyclable performance over 13 cycles. The effect of applied voltages was studied and it can be seen in Fig. 5(d) that higher voltages are favourable for electrosorption since the resulting electric field will be stronger. In addition, the increased voltage enables a more rapid rate of desalination and the resulting salt removal rates are 0.00708 mg g−1 s−1 at 1.0 V, 0.0108 mg g−1 s−1 at 1.2 V, 0.0229 mg g−1 s−1 at 1.4 V, 0.0262 mg g−1 s−1 at 1.6 V. The highest removal capacity of 18.88 mg g−1 occurred at 1.6 V which is an 81.7% improvement from 1.2 V. A summary of the electrosorption capacities of CHS 1, 3 and 5 in NaCl solutions of different concentrations is provided in Fig. S2(a).† There is a consistent improvement in electrosorption capacities for all CHS samples as concentration is increased due to a thinner EDL. CHS 1 exhibits the largest electrosorption capacity of 13.22 mg g−1 in a 750 mg L−1 NaCl solution at 1.2 V. Charge efficiency was also calculated and compared among the 3 samples. Charge efficiency relates the amount of energy to the desalination performance of the CDI cell and in an ideal scenario, a charge efficiency of 1 refers to the removal of one salt molecule per electron transferred from anode to cathode.6,40 In real systems, the charge efficiency is always less than 1 due to effects such as co-ion desorption. For our CHS samples, there is a general trend that the charge efficiency is higher for spheres of smaller sizes save for the value at 250 mg L−1 where CHS 3 is higher than CHS 1.
Empirical studies were also performed to quantify the experimental data with the use of appropriate models. Two types of adsorption isotherms (Langmuir and Freundlich) have been applied to our experimental data and the Langmuir isotherm was found to fit better. The Langmuir isotherm is given by:
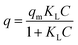 | (3) |
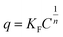 | (4) |
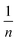 expresses the likelihood of adsorbate adsorption. The fitting curves are shown in Fig. 6 and the coefficients obtained from the fitting are listed in Table 2. The regression coefficients are for both Langmuir and Freundlich isotherms are close to 1 which indicates a good fit with the experimental data. According to the Langmuir isotherm, CHS 1 exhibits a maximum adsorption capacity of 15.24 mg g−1 which is 47.39% higher than CHS 3 and 71% greater than CHS 5. The kinetics of adsorption was investigated using Lagergren's pseudo first-order equation which is shown below:
expresses the likelihood of adsorbate adsorption. The fitting curves are shown in Fig. 6 and the coefficients obtained from the fitting are listed in Table 2. The regression coefficients are for both Langmuir and Freundlich isotherms are close to 1 which indicates a good fit with the experimental data. According to the Langmuir isotherm, CHS 1 exhibits a maximum adsorption capacity of 15.24 mg g−1 which is 47.39% higher than CHS 3 and 71% greater than CHS 5. The kinetics of adsorption was investigated using Lagergren's pseudo first-order equation which is shown below:
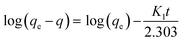 | (5) |
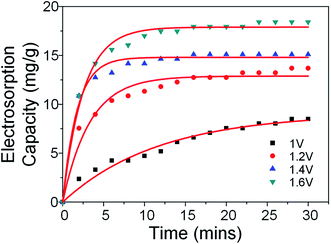 | ||
| Fig. 7 Electrosorption capacities of CHS 1 as a function of time and voltage. Red lines depict 1st order kinetic fitting. | ||
4. Conclusion
To conclude, carbon hollow spheres with mesoporous characteristics are synthesized through a modified Stöber method that combines both polymerization of the organic layer and the formation of the silica template in a single step. The size of the spheres is varied by addition of different amounts of TEOS precursor and these spheres are characterized in terms of their morphology, textural properties, electrochemical behaviours and CDI performances. It is found that a smaller sphere size is favourable for efficient desalination of saline water. CHS 1 has the smallest size of 230 nm and possesses a BET surface area of 809.91 m2 g−1 which is comparable to previous works on carbon spheres for CDI.3,29,42–45 The specific capacitance obtained in a 1 M NaCl at 10 mV s−1 was 191.15 F g−1 and a high electrosorption capacity of 18.88 mg g−1 was achieved at 1.6 V in a 250 mg L−1 NaCl solution. It is interesting to note that the development of mesoporosity only occurred for spheres of smaller sizes (in this case, <503 nm) and more detailed studies are required to fully understand the synthesis conditions that are responsible for this.Acknowledgements
The research project is supported by the Singapore National Research Foundation under its Environmental & Water Technologies Strategic Research Programme and administered by the Environment & Water Industry Programme Office (EWI) of the PUB.References
- H. S. Wheater and P. Gober, Water Resour. Res., 2015, 51, 5406–5424 CrossRef.
- C. M. Brown, J. R. Lund, X. Cai, P. M. Reed, E. A. Zagona, A. Ostfeld, J. Hall, G. W. Characklis, W. Yu and L. Brekke, Water Resour. Res., 2015, 51, 6110–6124 CrossRef.
- Y. Liu, L. Pan, T. Chen, X. Xu, T. Lu, Z. Sun and D. H. C. Chua, Electrochim. Acta, 2015, 151, 489–496 CrossRef CAS.
- S. Miller, H. Shemer and R. Semiat, Desalination, 2015, 366, 2–8 CrossRef CAS.
- M. A. Anderson, A. L. Cudero and J. Palma, Electrochim. Acta, 2010, 55, 3845–3856 CrossRef CAS.
- S. Porada, R. Zhao, A. van der Wal, V. Presser and P. M. Biesheuvel, Prog. Mater. Sci., 2013, 58, 1388–1442 CrossRef CAS.
- M. E. Suss, S. Porada, X. Sun, P. M. Biesheuvel, J. Yoon and V. Presser, Energy Environ. Sci., 2015, 8, 2296–2319 CAS.
- B. S. Shankar, K. P. Pranav and R. K. Raj, Capacitive Deionization based Water Desalination System using an MPPT based Solar Charge Controller, IEEE, 2014 Search PubMed.
- Y. Liu, C. Nie, X. Liu, X. Xu, Z. Sun and L. Pan, RSC Adv., 2015, 5, 15205–15225 RSC.
- L. Chao, Z. Liu, G. Zhang, X. Song, X. Lei, M. Noyong, U. Simon, Z. Chang and X. Sun, J. Mater. Chem. A, 2015, 3, 12730–12737 CAS.
- C.-L. Yeh, H.-C. Hsi, K.-C. Li and C.-H. Hou, Desalination, 2015, 367, 60–68 CrossRef CAS.
- H. Wang, T. Yan, P. Liu, G. Chen, L. Shi, J. Zhang, Q. Zhong and D. Zhang, J. Mater. Chem. A, 2016, 4, 4908–4919 CAS.
- Z. Li, B. Song, Z. Wu, Z. Lin, Y. Yao, K.-S. Moon and C. P. Wong, Nano Energy, 2015, 11, 711–718 CrossRef CAS.
- L. Chang, J. Li, X. Duan and W. Liu, Electrochim. Acta, 2015, 176, 956–964 CrossRef CAS.
- S. Zhao, T. Yan, H. Wang, G. Chen, L. Huang, J. Zhang, L. Shi and D. Zhang, Appl. Surf. Sci., 2016, 369, 460–469 CrossRef CAS.
- K.-H. Park and D.-H. Kwak, J. Electroanal. Chem., 2014, 732, 66–73 CrossRef CAS.
- J.-H. Choi, Sep. Purif. Technol., 2010, 70, 362–366 CrossRef CAS.
- M.-W. Ryoo and G. Seo, Water Res., 2003, 37, 1527–1534 CrossRef CAS PubMed.
- Y. Wang, X. Han, R. Wang, S. Xu and J. Wang, Electrochim. Acta, 2015, 182, 81–88 CrossRef CAS.
- X. Xu, Y. Liu, T. Lu, Z. Sun and L. Pan, J. Mater. Chem. A, 2015, 3, 13418–13425 CAS.
- Z. Wang, B. Dou, L. Zheng, G. Zhang, Z. Liu and Z. Hao, Desalination, 2012, 299, 96–102 CrossRef CAS.
- X. Xu, L. Pan, Y. Liu, T. Lu and Z. Sun, J. Colloid Interface Sci., 2015, 445, 143–150 CrossRef CAS PubMed.
- H. Jiang, J. Ma and C. Li, Adv. Mater., 2012, 24, 4197–4202 CrossRef CAS PubMed.
- C. Tsouris, R. Mayes, J. Kiggans, K. Sharma, S. Yiacoumi, D. DePaoli and S. Dai, Environ. Sci. Technol., 2011, 45, 10243–10249 CrossRef CAS PubMed.
- Z. Peng, D. Zhang, L. Shi and T. Yan, J. Mater. Chem., 2012, 22, 6603–6612 RSC.
- X. Gu, Y. Yang, Y. Hu, M. Hu, J. Huang and C. Wang, J. Mater. Chem. A, 2015, 3, 5866–5874 CAS.
- J. Liu, N. P. Wickramaratne, S. Z. Qiao and M. Jaroniec, Nat. Mater., 2015, 14, 763–774 CrossRef CAS PubMed.
- M. Chen, C. Yu, S. Liu, X. Fan, C. Zhao, X. Zhang and J. Qiu, Nanoscale, 2015, 7, 1791–1795 RSC.
- Y. Liu, T. Chen, T. Lu, Z. Sun, D. H. C. Chua and L. Pan, Electrochim. Acta, 2015, 158, 403–409 CrossRef CAS.
- H. Wang, L. Shi, T. Yan, J. Zhang, Q. Zhong and D. Zhang, J. Mater. Chem. A, 2014, 2, 4739–4750 CAS.
- I. A. Ibrahim, A. Zikry and M. A. Sharaf, Journal of American Science, 2010, 6, 985–989 Search PubMed.
- J. Liu, S. Z. Qiao, H. Liu, J. Chen, A. Orpe, D. Zhao and G. Q. M. Lu, Angew. Chem., Int. Ed., 2011, 50, 5947–5951 CrossRef CAS PubMed.
- A. B. Fuertes, P. Valle-Vigon and M. Sevilla, Chem. Commun., 2012, 48, 6124–6126 RSC.
- A. H. Lu, G. P. Hao and Q. Sun, Angew. Chem., Int. Ed., 2011, 50, 9023–9025 CrossRef CAS PubMed.
- I. Childres, L. A. Jauregui, W. Park, H. Cao and Y. P. Chen, New Dev. Photon Mater. Res., 2013, 978–981 Search PubMed.
- L.-H. Kao and T.-C. Hsu, Mater. Lett., 2008, 62, 695–698 CrossRef CAS.
- P. K. Shen, C.-Y. Wang, S. P. Jiang, X. Sun and J. Zhang, Electrochemical Energy: Advanced Materials and Technologies, CRC Press, 2015 Search PubMed.
- K.-L. Yang, T.-Y. Ying, S. Yiacoumi, C. Tsouris and E. S. Vittoratos, Langmuir, 2001, 17, 1961–1969 CrossRef CAS.
- C.-H. Hou, PhD Thesis, Georgia Institute of Technology, 2008.
- R. Zhao, P. M. Biesheuvel, H. Miedema, H. Bruning and A. van der Wal, J. Phys. Chem. Lett., 2010, 1, 205–210 CrossRef CAS.
- M. Mossad and L. Zou, Chem. Eng. J., 2013, 223, 704–713 CrossRef CAS.
- H. Li, S. Liang, J. Li and L. He, J. Mater. Chem. A, 2013, 1, 6335–6341 CAS.
- K. Laxman, M. T. Z. Myint, H. Bourdoucen and J. Dutta, ACS Appl. Mater. Interfaces, 2014, 6, 10113–10120 CAS.
- H. Wang, D. Zhang, T. Yan, X. Wen, J. Zhang, L. Shi and Q. Zhong, J. Mater. Chem. A, 2013, 1, 11778–11789 CAS.
- W. Shi, H. Li, X. Cao, Z. Y. Leong, J. Zhang, T. Chen, H. Zhang and H. Y. Yang, Sci. Rep., 2016, 6, 18966 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra06489b |
| This journal is © The Royal Society of Chemistry 2016 |