Improved H2 uptake capacity of transition metal doped benzene by boron substitution
Amol Deshmukhab,
Ravinder Kondacd,
Vijayanand Kalamsee and
Ajay Chaudhari*d
aInstitute of Atomic and Molecular Sciences, Molecular Science and Technology Program, Taiwan International Graduate Program, Academia Sinica, Taipei 11529, Taiwan
bDepartment of Physics, National Central University, Jung-Li 32001, Taiwan
cSchool of Physical Sciences, S. R. T. M. University, Nanded-431606, India
dDept. of Physics, The Institute of Science, Fort, Mumbai-400032, India. E-mail: ajaychau5@yahoo.com
eShri Guru Gobind Singhji Institute of Engineering & Technology, Nanded-431606, India
First published on 6th May 2016
Abstract
The effect of boron substitution on hydrogen storage capacity of transition metal (TM) doped benzene is studied using density functional theory and the second order Møller–Plesset method with aug-cc-pVDZ basis set. Out of the six carbon atoms in a benzene ring, two are substituted by boron atoms. The structures considered here are C4B2H6TM (TM = Sc, Ti, V). Four, four and three H2 molecules can be adsorbed on unsubstituted C6H6Sc, C6H6Ti and C6H6V complexes, respectively, whereas upon boron substitution one additional H2 molecule gets adsorbed on each of these complexes. The H2 uptake capacity of C4B2H6Sc, C4B2H6Ti and C4B2H6V obtained is 7.71, 7.54 and 5.99 wt%, respectively. Gibbs free energy corrected adsorption energies show that H2 adsorption on C4B2H6Sc is energetically unfavorable whereas it is favorable on C4B2H6Ti and C4B2H6V at ambient conditions. Various interaction energies for the H2 adsorbed complexes are obtained using a many-body analysis technique. The H2 desorption temperature for boron substituted TM doped benzene is lower than that for TM doped benzene for all the three systems. Molecular dynamics simulations show that loosely bonded H2 molecules in C4B2H6Sc(5H2) and C4B2H6Ti(5H2) complexes fly away during the simulation, thereby showing lower H2 uptake capacity of these complexes than that obtained by electronic structure calculations.
Introduction
Hydrogen is one of the best alternatives to fossil fuels. Obtaining suitable materials for storing hydrogen with high uptake capacity is the big obstacle for the hydrogen economy to be a reality.1,2 Materials that can store hydrogen with high gravimetric and volumetric density, exhibiting fast hydrogen sorption kinetics and that operate under ambient thermodynamic conditions are essential for practical applications, in particular for the transportation sector. Therefore, the search for promising hydrogen storage systems has attracted great attention. Practical hydrogen storage for vehicular applications requires materials with high hydrogen capacity, low decomposition temperature and fast adsorption/desorption kinetics. Hydrogen can be stored either in gaseous or in liquid forms in cryogenic tanks. The gaseous hydrogen storage requires high pressure tanks and liquid hydrogen storage requires very low temperature about −252.8 °C. During the required liquefaction process a significant amount of energy is consumed. Continuous efforts are made to store hydrogen at ambient conditions using solid state materials. Among the present hydrogen storage options, solid-state storage would satisfy the market requirement.3,4 Till date, no reversible materials are currently known that possess all mentioned attributes.Organometallic complexes are increasingly important in the hydrogen storage and hydrogen fuel cell sector. Recent research has demonstrated that some transition metal (TM) based organic materials can store hydrogen at levels exceeding those found in most materials currently being evaluated for hydrogen storage applications. Hydrogen storage on several small organometallic complexes have been tested. Hydrogen storage on transition metal–acetylene5–9 and transition metal–ethylene organometallic complexes8,10–20 has been studied thoroughly. Wadnerkar et al. have compared the hydrogen uptake capacity and equilibrium isotope effect from theory and experiment for the Ti:C2H4 organometallic complex.19 They have shown that Ti:C2H4 organometallic thin films can be synthesized in an ultra-high vacuum chamber using the method of reactive pulsed laser deposition. The equilibrium isotope effect calculated using vibrational frequencies and H2 uptake capacity of Ti:C2H4 from quantum chemical calculations is in excellent agreement with the experimental determinations.19 Phillips et al. have reported hydrogen adsorption on nanoscale titanium–benzene complex formed through reactive pulsed laser deposition in an ultra-high vacuum chamber.21 This has encouraged the researchers to study the H2 uptake capacity of different small organometallic complexes.
Several investigations have also been made on hydrogen storage capacity of TM–CnHn rings and TM–CnHm (ref. 21–30) complexes. The H2 uptake capacity of these complexes is as per the target set by the U.S. Department of Energy (US DOE). Though small organometallic complexes show high H2 uptake capacity satisfying the target set by US DOE, their H2 uptake capacity may be lower in bulk due to clustering of transition metal atoms. Therefore, continuous efforts are made to increase the H2 uptake capacity of small organometallic complexes containing single TM atom so that even if in bulk it decreases due to clustering of TM atoms it satisfies the target set by US DOE. From this point of view instead of transition metal atom alkali or alkaline earth metal atom decorated complexes are considered. However, it is observed that H2 adsorption on such complexes is energetically unfavorable at ambient conditions.8,9 Hence TM doped complexes are the best options for hydrogen storage, but we have to increase their H2 uptake capacity. Some efforts are made earlier to increase the hydrogen uptake capacity or metal binding energy by boron substitution. Lu et al. have investigated the effect of boron substitution in aromatic rings of graphyne for Li and H2 binding using Density Functional Theory (DFT), first principles molecular dynamics and grand canonical ensemble Monte Carlo method.31 Wang et al. have constructed three dimensional B-doped graphene-interconnected framework and reported 5.9 wt% hydrogen storage capacity of Li decorated material at 298 K and 100 bars.32 Using first principles calculations Lee et al. have reported hydrogen uptake capacity of Ca decorated zigzag graphene nanoribbon as 5 wt%.33 They concluded that on the zigzag edge and B-doped armchair edge of graphene Ca clustering is suppressed. Rao et al. using DFT calculations have shown that in porous graphene nanotube and B-substituted porous graphene nanotube both, the curvature effect and B substitution strengthen the Li, Ca and Na metal binding and prevent the metal atoms from clustering.34 Lu et al. have obtained the hydrogen uptake capacity of 6.4 and 6.8 wt% for two boron substituted Li decorated porous graphene and two boron substituted Ca decorated porous graphene respectively.35 Kalamse et al. have studied interaction of molecular hydrogen with Li and Ti functionalized boron substituted and unsubstituted naphthalene using DFT method and concluded that Ti containing complexes are superior over Li containing complexes for hydrogen storage.36 Zou et al. also observed suppressing of Sc, Ti and Ca clustering on covalent organic frameworks after boron doping.37 This work is another effort to predict hydrogen storage properties of a metal decorated boron substituted material at ambient conditions.
In this work, first we have studied hydrogen storage capacity of C6H6TM (TM = Sc, Ti, V) complexes viz. C6H6Sc, C6H6Ti, C6H6V using quantum chemical methods. We then studied C4B2H6Sc, C4B2H6Ti and C4B2H6V complexes obtained by substituting two boron atoms in place of two carbon atoms in a benzene ring and studied their H2 uptake capacity. The aim of this substitution is to study whether boron substitution enhances the H2 uptake capacity of unsubstituted organometallic complexes. The Gibbs free energy corrected adsorption energies are obtained at different temperatures and pressures to know at what temperature and pressure range H2 adsorption on these complexes is energetically favorable. Molecular dynamics (MD) simulations were also carried out using atom centered density matrix propagation (ADMP). H2 desorption temperatures from these complexes are obtained using Van't Hoff equation.38 Many-body analysis technique has been used to study various interaction energies in H2 adsorbed complexes.39–43
Computational details
We optimized the geometries of unsubstituted TM doped benzene and boron substituted TM doped benzene using second order Møller–Plesset method and DFT with PBEPBE and B3LYP functionals along with aug-cc-pVDZ basis set. We then added H2 molecules one by one on each of these complexes and optimized the geometries. Molecular dynamics simulations were carried out using atom centered density matrix propagation.44 The time step (Δt) of ADMP–MD simulations was set at 0.2 fs. The temperature was maintained using the velocity scaling method during the ADMP-MD simulations. All calculations were performed using the Gaussian suite of programs.45The averaged adsorption energy without zero point energy correction (ΔE) is calculated as
| ΔE = {E[OM] + (n × E[H2]) − E[OM(nH2)]}/n |
The averaged adsorption energy with zero point energy correction (ΔEZPE) is calculated as
| ΔEZPE = {EZPE[OM] + (n × EZPE[H2]) − EZPE[OM(nH2)]}/n |
The averaged adsorption energy with Gibbs free energy correction (ΔEG) is calculated as
| ΔEG = {EG[OM] + (n × EG[H2]) − EG[OM(nH2)]}/n |
Various interaction energies for H2 adsorbed complexes are obtained using many-body analysis technique. All the energies reported here are corrected for the basis set superposition error.
Results and discussion
Fig. 1 shows optimized structures of hydrogen adsorbed transition metal (TM) doped benzene and hydrogen adsorbed boron substituted TM doped benzene at MP2/aug-cc-pvdz level of theory. Four, four and three H2 molecules can be adsorbed on C6H6Sc, C6H6Ti and C6H6V organometallic complex respectively. When two of the carbon atoms in a benzene ring are substituted by boron atoms, these complexes adsorb one extra H2 molecule. The energy required to substitute two boron atoms in a benzene ring is found to be −8.5, −9.1 and −9.2 eV at PBEPBE, B3LYP and MP2 method respectively with aug-cc-pvdz basis set. The negative values indicate that substitution of boron in benzene is endothermic process and requires energy for substitution.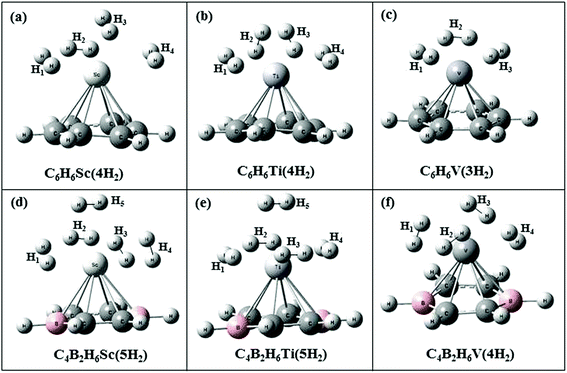 | ||
| Fig. 1 Optimized structures of (a) C6H6Sc(4H2) (b) C6H6Ti(4H2) (c) C6H6V(3H2) (d) C4B2H6Sc(5H2) (e) C4B2H6Ti(5H2) (f) C4B2H6V(4H2) at MP2/aug-cc-pvdz level. | ||
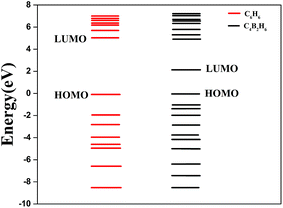
The H2 uptake capacity of C6H6Sc, C6H6Ti, C6H6V, C4B2H6Sc, C4B2H6Ti and C4B2H6V complexes is found to be 6.15, 6.02, 4.48, 7.71, 7.54 and 5.99 wt% respectively. Thus boron substitution has enhanced the H2 uptake capacity of C6H6Sc, C6H6Ti, C6H6V complexes by about 1.56, 1.52, and 1.51 wt% respectively. Substituting boron for carbon in benzene ring introduces new states above the highest occupied molecular orbital as shown in Fig. 2. This is in agreement with what observed by Park et al.46 They have shown that in boron substituted graphene-like structure, new states appear above the valence band maximum. This electron deficient structure accepts extra electrons and suppresses TMs clustering by increasing metal binding energy. Hindering metal clustering is one of the important issues for hydrogen storage on transition metal decorated materials because it not only affects the surface stability but reduces the surface area for hydrogen adsorption. Here, it is difficult to observe metal clustering because we are using only one TM atom on hexagonal site of C6H6 and C4B2H6 as a H2 interacting species. But, the multiple TM decorated complexes are more than likely to form cluster during synthesis. One way to understand the metal clustering thermodynamically is to compare metal binding energy on surface with its cohesive energy in bulk. In Table 1, we have compared Sc, Ti and V binding energy on C6H6 and C4B2H6 before and after H2 adsorption. The binding energy of Sc, Ti and V to C6H6(C4B2H6) is found to be 0.91(3.63), 1.39(4.8) and 1.72(5.57) eV respectively before H2 adsorption. It indicates that the TM atom bound strongly to boron substituted benzene than benzene. The binding energy of Sc, Ti and V to C4B2H6 in H2 adsorbed complexes is found to be 3.28, 4.38 and 3.87 respectively and little lower than that before H2 adsorption.
Calculated TM binding energies on C6H6 are much lower than the cohesive energy of TM in bulk. Consequently clustering of TM on C6H6 is likely to be happened. On the other hand, boron substituted benzene shows significant enhancement in TM binding energy as compared with un-substituted benzene. Calculated binding energies of TM on C4B2H6 before and even after H2 adsorption are found to be very close to their cohesive energy in bulk. Therefore, it can be concluded that B-substitution not only enhances hydrogen uptake but also helps to prevent the metal clustering. This is due to the fact that boron has one less valence electron than that for the carbon and when we substitute two of the carbon atoms with boron, C4B2H6 becomes an electron deficient surface with respect to C6H6. This forces TM to give some extra electron to C4B2H6 compared with C6H6. Table 2 shows calculated NBO charges for the H2 adsorbed complexes at MP2/aug-cc-pVDZ level.
| Complex | C | B | Metal |
|---|---|---|---|
| C6H6Sc | −0.41(−0.33) | — | 0.84(0.37) |
| C6H6Ti | −0.28(−0.25) | — | 0.60(−0.29) |
| C6H6V | −0.29(−0.25) | — | 0.45(−0.40) |
| C4B2H6Sc | −0.70(−0.62) | 0.20(0.28) | 1.72(0.86) |
| C4B2H6Ti | −0.77(−0.59) | 0.36(0.41) | 1.63(−0.1) |
| C4B2H6V | −0.66(−0.55) | 0.19(0.36) | 1.56(0.04) |
It is found that substitution of two boron atoms in place of carbon in benzene ring not only shows a prominent effect on metal binding but also on hydrogen adsorption. Table 3 shows a comparison of structural parameters for TM doped benzene and boron substituted TM doped benzene before H2 adsorption. As can be seen from Table 2, the C–C bond length gets little elongated on boron substitution for C6H6Sc and C6H6Ti whereas there is almost no change for the C6H6V complex. There is no large change in C–TM bond length before and after boron substitution. The C–B bond length is found to be 1.53 Å for all the three complexes. The B–TM bond lengths are longer than the C–TM bond lengths for all the three complexes. The B–TM and C–TM bond length decreases as we go from C4B2H6Sc to C4B2H6V complex.
| Complex | Bond length (Å) | |||
|---|---|---|---|---|
| C–C | C–B | C–TM | B–TM | |
| C6H6Sc | 1.41 | 1.53 | 2.35 | 2.47 |
| C4B2H6Sc | 1.44 | 2.35 | ||
| C6H6Ti | 1.44 | 1.53 | 2.14 | 2.38 |
| C4B2H6Ti | 1.48 | 2.15 | ||
| C6H6V | 1.44 | 1.53 | 2.10 | 2.32 |
| C4B2H6V | 1.43 | 2.14 | ||
Structural parameters for H2 adsorbed boron substituted TM doped benzene complexes at MP2/aug-cc-pVDZ, B3LYP/aug-cc-pVDZ and PBEPBE/aug-cc-pVDZ levels are compared in Table 4. The structural parameters at PBEPBE/aug-cc-pvdz level are comparable with those obtained at MP2/aug-cc-pvdz level. The C–C bond lengths in H2 adsorbed TM doped benzene complexes before and after boron substitution are equal. Similarly C–B bond lengths in boron substituted TM doped benzene before and after maximum H2 adsorption are also equal. There is large change in the C–TM bond lengths before and after H2 adsorption for unsubstituted as well as boron substituted TM doped benzene for all the three cases. The elongation of C–TM bond lengths after H2 adsorption indicates weakening of the C–TM bond after maximum H2 adsorption. The C–TM bond lengths for H2 adsorbed boron substituted TM doped benzene are longer than those for the H2 adsorbed unsubstituted TM doped benzene. The bond between boron and transition metal also becomes weak after the maximum number of H2 adsorption.
| Method | Bond length (Å) | |||||
|---|---|---|---|---|---|---|
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C |
C–B | C–TM | B–TM | TM–H2 | ||
| PBEPBE | a | 1.43(1.45) | 1.54(1.53) | 2.45(2.30) | 2.55(2.43) | 2.20(top), 2.14, 2.09, 2.08, 2.25 |
| b | 1.43(1.50) | 1.54(1.54) | 2.38(2.10) | 2.50(2.35) | 2.12(top), 1.86, 1.86, 1.89, 1.89 | |
| c | 1.42(1.43) | 1.54(1.53) | 2.29(2.24) | 2.40(2.33) | 1.78, 1.78, 1.81, 1.81 | |
| B3LYP | a | 1.42(1.44) | 1.53(1.53) | 2.46(2.34) | 2.56(2.47) | 2.30(top), 2.17, 2.13, 2.13, 2.31 |
| b | 1.42(1.47) | 1.52(1.52) | 2.37(2.13) | 2.51(2.37) | 2.37(top), 1.89, 1.89, 1.93, 1.93 | |
| c | 1.41(1.43) | 1.53(1.53) | 2.32(2.24) | 2.43(2.32) | 1.85, 1.83, 1.86, 1.83 | |
| MP2 | a | 1.43(1.45) | 1.53(1.53) | 2.44(2.39) | 2.55(2.51) | 2.36(top), 2.17, 2.23, 2.09, 2.15 |
| b | 1.43(1.50) | 1.54(1.54) | 2.36(2.10) | 2.50(2.36) | 2.13(top), 1.88, 1.88, 1.83, 1.83 | |
| c | 1.41(1.44) | 1.53(1.54) | 2.32(2.24) | 2.43(2.34) | 1.86, 1.83, 1.86, 1.83 | |
Boron substitution not only affects the bond length, but also the orientation of adsorbed H2 molecules. In case of C6H6Sc all the four H2 molecules are adsorbed with H–H bond parallel to the plane of benzene ring. Boron substitution results in adsorption of an extra H2 molecule which adsorbs on top of the Sc atom. Out of five adsorbed H2 molecules including the H2 molecule adsorbed on top of Sc atom three are having their H–H bond parallel to the plane of boron substituted benzene ring. For the remaining two, the H–H bond is perpendicular to the plane of boron substituted benzene ring. In case of C6H6Ti all the four H2 molecules are adsorbed with their H–H bond inclined to the plane of benzene ring. Upon boron substitution all the five H2 molecules are adsorbed with their orientation parallel to the plane of boron substituted benzene ring. For C6H6V complex all the three H2 molecules are adsorbed with their orientation parallel to the plane of benzene ring whereas upon boron substitution the adsorbed H2 molecules are with their orientation inclined to the boron substituted benzene ring. The H2 molecules are adsorbed at a little longer distance in boron substituted TM doped benzene than that for the TM doped benzene. This indicates weak interaction between H2 molecules and boron substituted TM doped benzene than that between H2 molecule and TM doped benzene.
Table 4 shows that for C4B2H6Sc(5H2) and C4B2H6Ti(5H2) complexes one of the five H2 molecules is adsorbed at a longer distance as compared to the remaining four adsorbed H2 molecules. It indicates that the fifth H2 molecule is loosely bonded to these complexes. For C4B2H6V complex all the four adsorbed H2 molecules are adsorbed at equal distance. The H–H bond lengths of adsorbed H2 molecules are longer than that for the isolated H2 molecule for all the H2 adsorbed complexes. The H–H bond length for the isolated H2 molecule is found to be 0.75 Å at MP2/aug-cc-pvdz level whereas it is found to be in a range of 0.78–0.79, 0.79–0.86 and 0.83–0.85 for C4B2H6Sc(5H2), C4B2H6Ti(5H2) and C4B2H6V(4H2) complexes respectively. The elongation in H–H bond length upon adsorption indicates that there is Kubas interaction between H2 molecules and TM atom. It also indicates that there is a charge transfer from d orbitals of TM atom to the H2 molecules. More the charge transfer more is the elongation. Number of H2 molecules adsorbed depends on number of empty d orbitals of TM. The charge transfer between sigma bonding orbitals of H2 to d orbitals of TM plays an important role in H2 interaction. Natural bond orbital analysis shows that boron substituted benzene ring gains extra 0.25, 0.35 and 0.1 electrons from Sc, Ti and V respectively as compared to that for the unsubstituted benzene ring. This is in agreement with earlier findings.46 This results in more empty d orbitals of TM to interact with one extra H2 molecules for boron substituted TM doped benzene.
Calculated averaged H2 adsorption energies without (ΔE), with zero point energy correction (ΔEZPE) and with Gibbs free energy correction (ΔEG) at 298.15 K and 1 atm pressure are presented in Table 5 for maximum H2 adsorbed complexes. As can be seen from Table 5, H2 adsorption on C6H6Sc and C4B2H6Sc complexes is energetically unfavourable at ambient conditions whereas it is favourable on C6H6Ti, C4B2H6Ti, C6H6V and C4B2H6V complexes. It can also be seen from Table 5 that the zero point energy correction for adsorption energy is significant and not negligible. The H2 adsorption energies for boron substituted TM doped benzene are lower than that for the TM doped benzene for all the three systems indicating weak interaction of H2 molecules with the former than the latter. Similar to the structural parameters H2 adsorption energies obtained by PBEPBE/aug-cc-pVDZ level are comparable with those from MP2/aug-cc-pVDZ level.
| Complex | PBEPBE | B3LYP | MP2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ΔE | ΔEZPE | ΔEG | ΔE | ΔEZPE | ΔEG | ΔE | ΔEZPE | ΔEG | |
| C6H6Sc(4H2) | 0.48 | 0.34 | 0.05 | 0.36 | 0.22 | −0.07 | 0.34 | 0.18 | −0.12 |
| C4B2H6Sc(5H2) | 0.37 | 0.23 | −0.06 | 0.29 | 0.16 | −0.12 | 0.33 | 0.18 | −0.09 |
| C6H6Ti(4H2) | 0.73 | 0.55 | 0.26 | 0.64 | 0.45 | 0.16 | 0.76 | 0.59 | 0.29 |
| C4B2H6Ti(5H2) | 0.59 | 0.41 | 0.13 | 0.47 | 0.31 | 0.01 | 0.70 | 0.53 | 0.23 |
| C6H6V(3H2) | 0.89 | 0.73 | 0.44 | 0.78 | 0.60 | 0.31 | 1.49 | 1.27 | 0.96 |
| C4B2H6V(4H2) | 0.77 | 0.56 | 0.26 | 0.56 | 0.39 | 0.08 | 0.73 | 0.52 | 0.21 |
Since adsorption of five H2 molecules on C4B2H6Sc is energetically unfavorable at ambient conditions we have calculated H2 adsorption energies at different temperatures and pressures to know the suitable temperature and pressure range over which H2 adsorption on C4B2H6Sc, C4B2H6Ti and C4B2H6V is energetically favorable. For obtaining H2 adsorption energies at different temperatures, the pressure is kept constant as 1 atm. For calculating H2 adsorption energies at different pressures, the temperature is kept constant as 298.15 K. Fig. 3 and 4 show temperature and pressure dependent H2 adsorption energies respectively for C4B2H6Sc(5H2), C4B2H6Ti(5H2) and C4B2H6V(4H2) complexes. As observed earlier for structural parameters and H2 adsorption energies, temperature and pressure dependent H2 adsorption energies at PBEPBE/aug-cc-pVDZ and MP2/aug-cc-pVDZ levels are comparable. Fig. 3 shows that H2 adsorption on C4B2H6Ti and C4B2H6V complexes is energetically favorable at all the temperatures considered here at MP2/aug-cc-pVDZ as well as PBEPBE/aug-cc-pVDZ level. H2 adsorption on C4B2H6Sc is possible below 200 and 250 K at MP2/aug-cc-pVDZ and PBEPBE/aug-cc-pVDZ levels respectively. H2 adsorption on C4B2H6Ti and C4B2H6V is possible for a wide range of temperature than that on C4B2H6Sc. Fig. 4 shows that maximum number of H2 adsorption on C4B2H6Ti and C4B2H6V complexes is possible at all the pressures considered here at MP2/aug-cc-pVDZ and PBEPBE/aug-cc-pVDZ levels. The Gibbs free energy corrected H2 adsorption energies are higher at lower temperature and 1 atm pressure. Similarly H2 adsorption energies are higher at higher pressure and room temperature. Higher Gibbs free energy corrected H2 adsorption energies at lower temperature and higher pressure indicates stronger interaction of H2 molecules with respective organometallic complex.
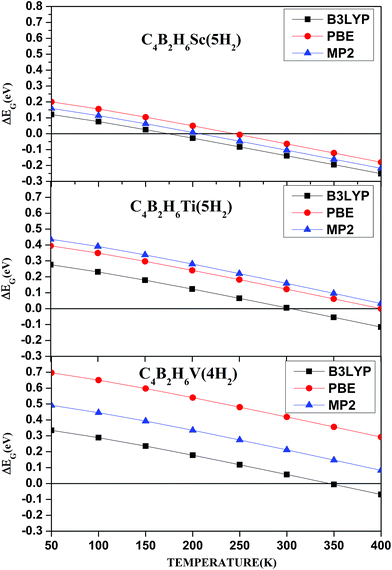 | ||
| Fig. 3 Temperature dependent Gibbs free energy corrected adsorption energies for C4B2H6Sc(5H2), C4B2H6Ti(5H2), and C4B2H6V(4H2) at different levels. | ||
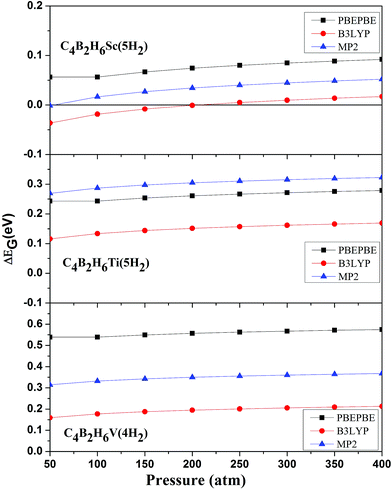 | ||
| Fig. 4 Pressure dependent Gibbs free energy corrected adsorption energies for C4B2H6Sc(5H2), C4B2H6Ti(5H2), and C4B2H6V(4H2) at different levels. | ||
The stability of H2 adsorbed complexes is confirmed by vibrational frequencies and the gap between Highest Occupied Molecular Orbital (HOMO) and Lowest Unoccupied Molecular Orbital (LUMO). The HOMO–LUMO gap with successive adsorption of H2 molecules for all the three complexes is shown in Fig. 5. It can be seen that the HOMO–LUMO gap for the maximum number of H2 adsorbed complex is greater than 6.5 eV at MP2/aug-cc-pVDZ level indicating that these complexes are kinetically stable. Also there are no soft vibrational modes for all the three H2 adsorbed complexes indicating quantum mechanically stable complexes. Also HOMO–LUMO gap for the H2 adsorbed complexes is higher than that for the respective isolated organometallic complexes at MP2/aug-cc-pVDZ level indicating more kinetic stability of former than the latter.
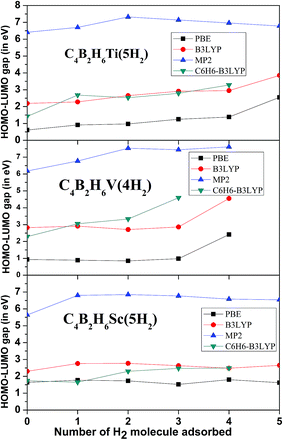 | ||
| Fig. 5 HOMO–LUMO gap with successive addition of H2 molecules on C4B2H6Sc, C4B2H6Ti, and C4B2H6V complex. | ||
Estimation of H2 desorption temperature from C6H6Sc, C4B2H6Sc, C6H6Ti, C4B2H6Ti, C6H6V and C4B2H6V complexes is obtained using the Van't Hoff equation38 written in terms of ΔEZPE
TD = (ΔEZPE/kB)(ΔS/R − ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P)−1 P)−1 |
Many-body analysis technique has been used here to obtain various interaction energies in H2 adsorbed complexes and are presented in Table 6. As can be seen from Table 6 two body interactions of adsorbed hydrogen molecules with all the three organometallic (OM) complexes are attractive whereas all the Hi–Hj two body interactions are repulsive. Here Hi and Hj are ith and jth hydrogen molecules in a complex respectively as shown in Fig. 1. The interaction energy depends on how far the hydrogen molecule is adsorbed in a complex. In case of C4B2H6Sc(5H2) and C4B2H6Ti(5H2) complexes the fifth and fourth hydrogen molecule is loosely bonded respectively to the respective OM complex and shows lower OM–Hi interaction energy as compared to that for the remaining hydrogen molecules within the same complex. In case of C4B2H6V(4H2) complex two hydrogen molecules are adsorbed at 1.842 Å and show equal OM–Hi interaction energy whereas the remaining two hydrogen molecules are adsorbed at 1.831 Å and the interaction energy for these two hydrogen molecules with C4B2H6V is also equal. For C4B2H6Sc(5H2) complex, first and fourth hydrogen molecules are adsorbed at equal distance of 2.124 Å and there is no large difference between the OM–Hi interaction energy for these two molecules with OM complex. Second and third hydrogen molecules are adsorbed at 2.283 Å and 2.170 Å respectively and result in little higher interaction energy for the latter than the former with OM complex. In case of C4B2H6Ti(5H2) complex first and second adsorbed hydrogen molecules are at 1.891 Å and having equal interaction energy with C4B2H6Ti complex. Similarly the third and fifth adsorbed hydrogen molecules are at equal distance of 1.926 Å and show equal two body interaction energy.
| Interaction terms | Interaction energy (kcal mol−1) | ||
|---|---|---|---|
| C4B2H6SC(5H2) | C4B2H6Ti(5H2) | C4B2H6V(4H2) | |
| Two body | |||
| OM–H1 | −9.31 | −20.86 | −10.70 |
| OM–H2 | −6.37 | −20.86 | −12.22 |
| OM–H3 | −7.48 | −17.83 | −12.22 |
| OM–H4 | −9.48 | −5.34 | −10.70 |
| OM–H5 | −2.83 | −17.82 | — |
| H1–H2 | 0.28 | 0.14 | 2.06 |
| H1–H3 | 0.24 | 1.72 | 4.52 |
| H1–H4 | 0.06 | 1.34 | 0.25 |
| H1–H5 | 1.19 | 1.72 | — |
| H2–H3 | 0.05 | 1.73 | 0.16 |
| H2–H4 | 1.21 | 1.34 | 4.51 |
| H2–H5 | 0.53 | 1.73 | — |
| H3–H4 | 0.29 | 2.04 | 2.05 |
| H3–H5 | 1.70 | 0.14 | — |
| H4–H5 | 0.58 | 2.05 | — |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Three body | |||
| OM–H1–H2 | 2.01 | 7.86 | −4.61 |
| OM–H1–H3 | 0.05 | −0.18 | −2.82 |
| OM–H1–H4 | 2.30 | −1.97 | 1.62 |
| OM–H1–H5 | −1.70 | −0.23 | — |
| OM–H2–H3 | 1.62 | −0.23 | −0.46 |
| OM–H2–H4 | −0.73 | −1.97 | −2.80 |
| OM–H2–H5 | 0.66 | −0.19 | — |
| OM–H3–H4 | −0.25 | −2.10 | −4.61 |
| OM–H3–H5 | −3.09 | 7.55 | — |
| OM–H4–H5 | −1.75 | −2.10 | — |
| H1–H2–H3 | −0.03 | −0.25 | −0.37 |
| H1–H2–H4 | −0.05 | −0.13 | −0.50 |
| H1–H2–H5 | −0.18 | −0.25 | — |
| H1–H3–H4 | −0.03 | −0.65 | −0.50 |
| H1–H3–H5 | −0.13 | −0.25 | — |
| H1–H4–H5 | −0.08 | −0.66 | — |
| H2–H3–H4 | −0.05 | −0.66 | −0.37 |
| H2–H3–H5 | −0.06 | −0.25 | — |
| H2–H4–H5 | −0.20 | −0.65 | — |
| H3–H4–H5 | −0.17 | −0.14 | — |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Four body | |||
| OM–H1–H2–H3 | −1.57 | −2.22 | −3.01 |
| OM–H1–H2–H4 | −0.13 | 1.47 | −3.36 |
| OM–H1–H2–H5 | −1.35 | −2.22 | — |
| OM–H1–H3–H4 | −0.75 | 2.49 | −3.35 |
| OM–H1–H3–H5 | 1.20 | −2.22 | — |
| OM–H1–H4–H5 | −1.05 | 2.47 | — |
| OM–H2–H3–H4 | 0.20 | 2.48 | −3.01 |
| OM–H2–H3–H5 | 0.02 | −2.22 | — |
| OM–H2–H4–H5 | 1.31 | 2.49 | — |
| OM–H3–H4–H5 | 1.13 | 3.92 | — |
| H1–H2–H3–H4 | 0.03 | 0.22 | 0.79 |
| H1–H2–H3–H5 | 0.03 | 0.48 | — |
| H1–H2–H4–H5 | 0.06 | 0.22 | — |
| H1–H3–H4–H5 | 0.03 | 0.22 | — |
| H2–H3–H4–H5 | 0.06 | 0.22 | — |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Five body | |||
| IOM–H1–H2–H3–H4 | −0.11 | −2.62 | 5.70 |
| IOM–H1–H2–H3–H5 | 0.70 | −0.15 | — |
| IOM–H1–H2–H4–H5 | −0.86 | −2.61 | — |
| IOM–H1–H3–H4–H5 | 0.34 | −3.89 | — |
| IOM–H2–H3–H4–H5 | −1.13 | −3.89 | — |
| H1–H2–H3–H4–H5 | −0.02 | −0.11 | — |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Six body | |||
| IOM–H1–H2–H3–H4–H5 | 0.75 | 3.89 | — |
| Sum of 2-body | −29.31 | −68.77 | −32.29 |
| Sum of 3-body | −1.88 | 2.56 | −15.42 |
| Sum of 4-body | −0.79 | 7.81 | −11.95 |
| Sum of 5-body | −1.07 | −13.26 | 5.70 |
| Sum of 6-body | 0.75 | 3.89 | — |
| Relaxation energy | −1.09 | 10.92 | 1.93 |
| Additive energy | −29.31 | −68.77 | −32.29 |
| Non additive energy | −2.98 | 1.01 | −21.67 |
| Binding energy | −33.39 | −56.84 | −52.03 |
| BSSE corrected total energy (Hartree) | −972.40 | −1061.18 | −1154.52 |
Similar to OM–Hi two body interaction energies, the Hi–Hj interaction energies within the same complex are also dependent on distance between Hi and Hj for all the three complexes. In C4B2H6Sc(5H2) complex the fifth hydrogen molecule is adsorbed on top of Sc atom. H1 is adsorbed on one side of Sc and H4 on another. Similar is the case for H2 and H3. The distance between H1–H2, H1–H3, H1–H4, H1–H5, H2–H3, H2–H4, H2–H5, H3–H4, H3–H5 and H4–H5 is found to be 2.272, 2.655, 4.118, 2.083, 4.128, 2.411, 2.441, 2.603, 2.322 and 2.255 Å respectively. The repulsive interaction energies between H1–H4 and H2–H3 molecules are almost negligible than that for other pairs of hydrogen molecules.
In case of C4B2H6Ti(5H2) complex, H1 and H2 adsorbed on opposite side of Ti. Similar is the case for H3 and H5. Two-body repulsive interaction energies for these two pairs are negligible as compared to other Hi–Hj interaction energies within the same complex. The distance between H1–H2, H1–H3, H1–H4, H1–H5, H2–H3, H2–H4, H2–H5, H3–H4, H3–H5 and H4–H5 is found to be 3.557, 1.932, 2.290, 1.932, 1.930, 2.289, 1.932, 2.389, 3.539, 3.539 and 2.388 Å respectively. As compared to C4B2H6Sc(5H2) and C4B2H6Ti(5H2) complexes, the Hi–Hj distances are shorter in C4B2H6V(4H2) complex and show higher Hi–Hj two body repulsive interaction energies. Here also H1 and H4 are adsorbed on opposite side of V. Similar is the case for H2 and H3. These two interaction energies are negligible as compared to other Hi–Hj two-body repulsive interaction energies within the same complex.
Total two-body interaction energy contributes about 87.8, 120.9 and 62.1% attractively to the binding energy of C4B2H6Sc(5H2), C4B2H6Ti(5H2) and C4B2H6V(4H2) complex respectively. The OM–Hi two-body interaction energies have 121, 120 and 142% attractive contribution whereas Hi–Hj interaction energies have 21, 20 and 42% repulsive contribution to the total two-body interaction energy for C4B2H6Sc(5H2), C4B2H6Ti(5H2) and C4B2H6V(4H2) complex respectively.
Not only two-body interaction energies but higher energies viz. three-body, four-body etc. and relaxation energies are also contributing significantly to the binding energy of a respective complex. For total three-body, total four-body, total five-body and total six-body energies, contribution from many-body energies containing OM complex as one of the many-body terms is more as compared to that from the terms containing only hydrogen molecules. The attractive% contribution from total three body, total four body and total five body energy to the binding energy of C4B2H6Sc(5H2) complex is 5.63, 2.4 and 3.2% respectively where as the only six body energy for this complex contributes about 2.2%.
For C4B2H6Ti(5H2) complex the repulsive% contribution from total three-body, total four-body and total six-body energy to its binding energy is 4.5, 13.7 and 6.84% respectively whereas total five body energy has 23.3% attractive contribution. In case of C4B2H6V(4H2) complex, total three body and total four body has about 29.6 and 22.9% attractive contribution to its binding energy whereas total five body energy contributes repulsively by about 11%. The binding energy of C4B2H6Sc(5H2), C4B2H6Ti(5H2) and C4B2H6V(4H2) complex is found to be 33.39, 56.84 and 52.03 kcal mol−1 respectively.
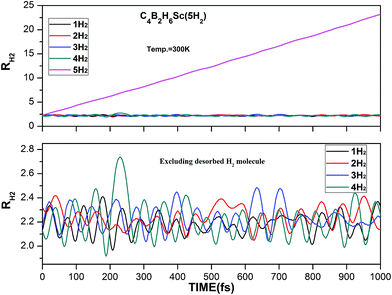
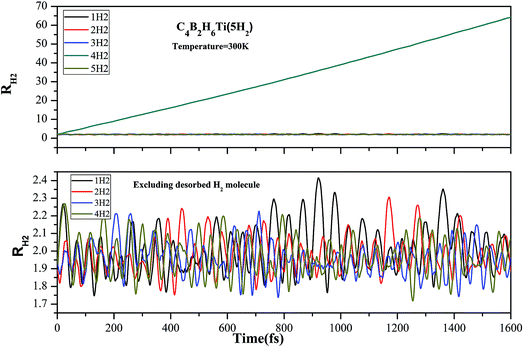
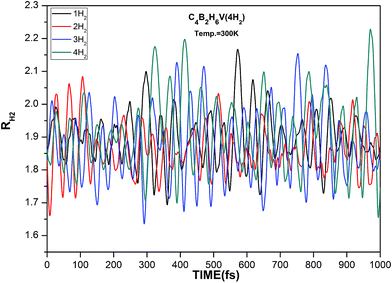
ADMP-MD simulations have been performed to confirm whether boron substituted TM decorated benzene complexes can adsorb hydrogen molecules at finite temperature. The minimum energy structures obtained using the electronic structure calculations have been used as initial geometries for the ADMP-MD simulations. The trajectories of H2 at 300 K for the three complexes are shown in Fig. 6–8. The TM–H2 distances in all the H2 adsorbed complexes studied here are shown in Table 4. The TM–H2 distance oscillation range (1.7–2.3 Å) in the MD simulations is reasonable to compare with optimized distance by different level of theory. No H2 molecule is oscillating far from their maximum distance observed in optimized structure. The labile H2 molecules leave very early (around 50 fs) from Sc and Ti decorated C4B2H6 complexes by leaving more space for other H2 molecules to interact with TM. This is true for other systems studied earlier as well.7,20,30 Other hydrogen molecules remain adsorbed on a complex during the simulation of 1000 fs or even longer. To confirm this we have performed ADMP-MD simulation for longer than 1000 fs for C4B2H6Ti(5H2) system upto 1600 fs and results are shown in Fig. 7. It can be concluded that the H2 molecules which are having a tendency to leave the complex, fly away very early and 1000 fs simulation time seems to be long enough.
From Fig. 6 and 7 out of five adsorbed H2 molecules in initial structure of C4B2H6Sc(5H2) and C4B2H6Ti(5H2) at least four hydrogen molecules are retained on Sc/Ti atom during the simulations. One of the five hydrogen molecules which was loosely bonded to the OM complex gets desorbed during the ADMP-MD simulations. During ADMP-MD simulations the four adsorbed hydrogen molecules on C4B2H6Sc(5H2) and C4B2H6Ti(5H2) oscillate at a distance in a range of 1.7 to 2.3 Å. In Fig. 7, it is observed that the first hydrogen molecule shows minor oscillations for Ti–H2 distance upto 500 fs after which that begins to grow in magnitude till 1000 fs. To confirm whether this hydrogen molecule remain adsorbed on C4B2H6Ti complex or not, we have performed ADMP-MD simulations for longer than 1000 fs up to 1600 fs. It is observed that Ti–H2 distance for this hydrogen molecule becomes shorter after 1000 fs. It indicates that it remain adsorbed on C4B2H6Ti complex during entire simulation period of 1600 fs and does not leave the complex.
In case of C4B2H6V(4H2) all the four molecules are remain adsorbed during the ADMP-MD simulations as can be seen in Fig. 8. This is due to the fact that all the four molecules are strongly bonded to the C4B2H6V complex and there is no loosely bonded hydrogen molecule for C4B2H6V(4H2) complex. From ADMP-MD simulations each of the complex C4B2H6Sc, C4B2H6Ti and C4B2H6V can retain four hydrogen molecules during the simulations thereby showing hydrogen uptake capacity of 6.26, 6.13 and 5.99 wt% respectively. The predicted hydrogen uptake capacity of boron substituted TM doped benzene from electronic structure calculations as well as ADMP-MD simulations for all the three complexes is satisfying the target set by US DOE.
Conclusions
Second order Møller–Plesset method, density functional theory with PBEPBE and B3LYP functionals and aug-cc-pVDZ basis set have been used to study the effect of boron substitution on hydrogen uptake capacity of Sc, Ti and V doped benzene. Boron substitution enhances the hydrogen uptake capacity of C6H6Sc, C6H6Ti and C6H6V by about 1.5 wt%. Electronic structure calculations show that five, five and four hydrogen molecules get adsorbed on C6B2H6Sc, C6B2H6Ti and C6B2H6V complex respectively. However ADMP-MD simulations show that four hydrogen molecules are remain adsorbed on C6B2H6Sc, C6B2H6Ti and C6B2H6V complexes during the simulation with respective hydrogen uptake capacity of 6.26, 6.13 and 5.99 wt%. Hydrogen desorption temperature is found to be lowered for the boron substituted TM doped benzene than that for the TM doped benzene. Two-body interaction energies show that hydrogen molecules interact strongly with C4B2H6Ti than C4B2H6Sc and C4B2H6V complexes. The hydrogen uptake capacity predicted here for the boron substituted TM doped benzene complexes is satisfying the target set by US DOE.Acknowledgements
Financial support from CSIR, New Delhi, India (Grant No: 03(1223)/12/EMR-II) is thankfully acknowledged.References
- Solid state hydrogen storage materials and chemistry, ed. G. Walker, Woodhead, 2008 Search PubMed.
- http://www.eere.energy.gov/hydrogenandfuelcells/mypp.
- D. P. Broom, Hydrogen Storage Materials The Characterisation of Their Storage Properties, Springer, 2011 Search PubMed.
- Handbook of Hydrogen Storage: New Materials for Future Energy Storage, ed. M. Hirscher, Wiley-VCH, 2010 Search PubMed.
- L. J. Ma, J. Jia, H. S. Wu and Y. Ren, Int. J. Hydrogen Energy, 2013, 38, 16185 CrossRef CAS.
- L. J. Ma, J. Jia and H. S. Wu, Int. J. Hydrogen Energy, 2015, 40, 420 CrossRef CAS.
- V. Kalamse, N. Wadnerkar, A. Deshmukh and A. Chaudhari, Int. J. Hydrogen Energy, 2012, 37, 3727 CrossRef CAS.
- V. Kalamse, N. Wadnerkar, A. Deshmukh and A. Chaudhari, Int. J. Hydrogen Energy, 2012, 37, 5114 CrossRef CAS.
- P. Tavhare, V. Kalamse, R. Bhosale and A. Chaudhari, Struct. Chem., 2015, 26, 823 CrossRef CAS.
- C. S. Liu and Z. Zeng, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 245419 CrossRef.
- E. Durgun, S. Ciraci, W. Zhou and T. Yildirim, Phys. Rev. Lett., 2006, 97, 226102–226111 CrossRef CAS PubMed.
- W. Zhou, T. Yildirim, E. Durgun and S. Cirac, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 085434–085439 CrossRef.
- N. Wadnerkar, V. Kalamse and A. Chaudhari, Theor. Chem. Acc., 2010, 127, 285 CrossRef CAS.
- A. Chakraborty, S. Giri and P. K. Chattaraj, Struct. Chem., 2011, 22, 823 CrossRef CAS.
- A. B. Phillips and B. S. Shivaram, Nanotechnology, 2009, 20, 204020 CrossRef CAS PubMed.
- Y. Okamoto, J. Phys. Chem. C, 2008, 112, 17721 CAS.
- N. He, T. Gao, Z. Zhang, X. Tian and H. Han, J. Mol. Struct.: THEOCHEM, 2009, 916, 147 CrossRef CAS.
- V. Kalamse, N. Wadnerkar and A. Chaudhari, J. Phys. Chem. C, 2010, 114, 4704 CAS.
- N. Wadnerkar, V. Kalamse, A. B. Phillips, B. S. Shivaram and A. Chaudhari, Int. J. Hydrogen Energy, 2011, 36, 9727 CrossRef CAS.
- N. Wadnerkar, V. Kalamse and A. Chaudhari, RSC Adv., 2012, 2, 8497 RSC.
- A. B. Phillips, B. S. Shivaram and G. R. Myneni, Int. J. Hydrogen Energy, 2012, 37, 1546 CrossRef CAS.
- N. Wadnerkar, V. Kalamse and A. Chaudhari, Int. J. Hydrogen Energy, 2011, 36, 664 CrossRef CAS.
- A. I. Martinez, J. Nano Res., 2009, 5, 113 CrossRef CAS.
- P. F. Wecka and T. J. D. Kumar, J. Chem. Phys., 2007, 126, 094703–94711 CrossRef PubMed.
- Z. Guo, W. Wu and H. Zhang, Struct. Chem., 2009, 20, 1107 CrossRef.
- B. Kiran, A. K. Kandalam and P. Jena, J. Chem. Phys., 2006, 124, 224703–224711 CrossRef PubMed.
- Q. Dong, W. Q. Tian, D. L. Chen and C. C. Sun, Int. J. Hydrogen Energy, 2009, 34, 5444 CrossRef CAS.
- G. Chen, P. Jena and Y. Kawazoe, J. Chem. Phys., 2008, 129, 074305 CrossRef CAS PubMed.
- N. Wadnerkar, V. Kalamse and A. Chaudhari, Struct. Chem., 2013, 24, 369 CrossRef CAS.
- N. Wadnerkar, V. Kalamse, S. L. Lee and A. Chaudhari, J. Comput. Chem., 2012, 33, 170 CrossRef CAS PubMed.
- R. Lu, D. Rao, Z. Meng, X. Zhang, G. Xu, Y. Liu, E. Kan, C. Xiao and K. Deng, Phys. Chem. Chem. Phys., 2013, 15, 16120 RSC.
- Y. Wang, Z. Meng, Y. Liu, D. you, K. Wu, J. Lu, X. Wang, K. Deng, D. Rao and R. Lu, Appl. Phys. Lett., 2015, 106, 063901 CrossRef.
- H. Lee, J. Ihm, M. L. Kohen and S. G. Louie, Nano Lett., 2010, 10, 793 CrossRef CAS PubMed.
- D. Rao, R. Lu, Z. Meng, Y. Wang, Z. Lu, Y. Liu, X. Chen, E. Kan, C. Xiao, K. Deng and H. Wu, Int. J. Hydrogen Energy, 2014, 39, 18966 CrossRef CAS.
- R. Lu, D. Rao, Z. Lu, J. Qian, F. Li, H. Wu, Y. Wang, C. Xiao, K. Deng, E. Kan and W. Deng, J. Phys. Chem. C, 2012, 116, 21291 CAS.
- V. Kalamse, R. Krishna, E. Titus and A. Chaudhari, Int. J. Hydrogen Energy, 2016 DOI:10.1016/j.ijhydene.2015.11.171.
- X. Zou, G. Zhou, W. Duan, K. Choi and K. Ihm, J. Phys. Chem. C, 2010, 114, 13402 CAS.
- W. Zhou, T. Yildirim, E. Durgun and S. Ciraci, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 085434 CrossRef.
- S. S. Xantheas, J. Chem. Phys., 1994, 100, 7523 CrossRef CAS.
- S. S. Xantheas, J. Chem. Phys., 1995, 102, 4505 CrossRef CAS.
- A. Chaudhari, P. K. Sahu and S. L. Lee, J. Chem. Phys., 2004, 120, 170 CrossRef CAS PubMed.
- A. Chaudhari and S. L. Lee, J. Chem. Phys., 2004, 120, 7464 CrossRef CAS PubMed.
- A. Kulkarni, V. Ganesh and S. R Gadre, J. Chem. Phys., 2004, 121, 5043 CrossRef CAS PubMed.
- H. B. Schlegel, S. S. Iyengar, X. Li, J. M. Millam, G. A. Voth and G. E. Scuseria, et al., J. Chem. Phys., 2002, 117, 8694 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb and J. R. Cheeseman, et al., Gaussian 09. Revision D.01, Gaussian Inc, Wallingford CT, 2009 Search PubMed.
- H. L. Park, S. C. Yi and Y. C. Chun, Int. J. Hydrogen Energy, 2010, 35, 3583 CrossRef CAS.
- D. R. Lide, CRC Handbook of Chemistry and Physics, New York, 75th edn, 1994 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |