A practical green chemistry approach to synthesize fused bicyclic 4H-pyranes via an amine catalysed 1,4-addition and cyclization cascade†
Abstract
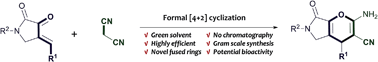
A newly developed synthetic approach to densely functionalized 4H-pyrane derivatives via an amine catalysed cascade reaction is presented. This protocol is relatively environmentally benign because it proceeds smoothly in water or ethanol at ambient temperature with low catalyst loading; more importantly, the products are easily purified, and some show promising antibacterial activity.

 Please wait while we load your content...
Please wait while we load your content...