Encapsulation of epoxy and amine curing agent in PAN nanofibers by coaxial electrospinning for self-healing purposes
Abstract
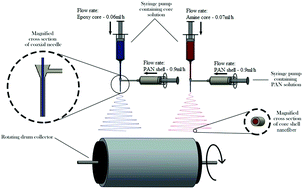
Dual components of a self-healing epoxy system comprising an epoxy resin and its amine based curing agent were encapsulated in a polyacrylonitrile (PAN) shell via a coaxial electrospinning technique. The morphological study showed the electrospun core–shell nanofibers were smooth, continuous, and without beads, with average diameters measured to be 483 nm and 406 nm for encapsulated epoxy and amine nanofibers, respectively. Investigations into the nanofiber's chemical structure showed the successful encapsulation of the epoxy resin and amine curing agent in the PAN shell, retaining the chemical characteristics of the encapsulated components. The thermal characterization of the nanofibers reinforced these findings, showing a 24 wt% and 37 wt% availability of the epoxy resin and amine curing agent contained within the PAN nanofibers, respectively. In addition, DSC results showed that this system holds great promise for self-healing epoxy resins in composite applications, particularly because of its spontaneous room temperature curing characteristics.

 Please wait while we load your content...
Please wait while we load your content...