W-shaped liquid crystalline dimers†
Martin Horčica,
Jiří Svoboda*a,
Arnošt Seidlera,
Václav Kozmíka,
Vladimíra Novotnáb,
Damian Pociechac and
Ewa Goreckac
aDepartment of Organic Chemistry, University of Chemistry and Technology, CZ-166 28 Prague 6, Czech Republic. E-mail: Jiri.Svoboda@vscht.cz; Fax: +420 220444182; Fax: +420 220444288
bInstitute of Physics, Czech Academy of Sciences, Na Slovance 2, CZ-182 21 Prague 9, Czech Republic. E-mail: novotna@fzu.cz; Fax: +420 286890527; Tel: +420 266053111
cLaboratory of Dielectrics and Magnetics, Chemistry Department, Warsaw University, Al. Zwirki i Wigury 101, 02-089 Warsaw, Poland. E-mail: pociu@chem.uw.edu.pl; Fax: +48 228221075; Tel: +48 228221075
First published on 13th April 2016
Abstract
We present a completely new structural type of W-shape dimers based on naphthalene-2,7-diol and 7-hydroxynaphthalene-2-carboxylic acid. First, bent-core monomers with a terminal hydroxyl group were designed and their mesomorphic properties established. Various modifications of the W-shape dimers were synthesised when two fully developed bent-core monomers were joined together with an isophthalic acid moiety, which introduced the additional inner molecular bend. The W-shape dimers demonstrated that their mesomorphic properties are more complex and versatile in comparison with the corresponding bent-core systems. Contrary to the corresponding monomers, most dimers are enantiotropic and reveal rich polymorphism. Even dimers created from non-mesogenic monomers exhibit mesophases, so the stabilising effect of the dimerisation process can be emphasised.
Introduction
Liquid crystals (LC) represent a unique state of matter, which links the anisotropy of the solid state and the fluidity of liquids. The molecular organisation and supramolecular interactions combine order and mobility at the molecular level and are the key features of the remarkable self-assembling properties at the nanoscale level. Among the many diverse structural types of liquid crystals, bent-core mesogens have become the subject of intensive investigation due to their unique properties, spontaneous polar order, and macroscopic chirality of smectic layers, despite their molecules being achiral.1–5 In addition to nematic and polar smectic phases, B1-type columnar mesophases have also been discovered for bent-core mesogens.6–8 The driving force is a polarisation splay, which generates different defect boundaries and a variety of 2D structures.Calamitic LC dimers and oligomers have been formerly investigated as convenient models to study the correlation between the molecular structure and mesophase formation for liquid crystalline main-chain and side-chain polymers.9–14 Research in this field is attracting increasing interest because the variability in the design and molecular structures of these materials is almost immense and as the mesomorphic properties of LC dimers are complex and different from the corresponding monomers. Generally, calamitic, discotic, and bent-core mesogens have been applied to create dimers of various shapes, such as H-,15–17 T-shaped18,19 and disc-shaped,20 but most of the dimers are derived from calamitic and bent-core mesogens. Recently, the twist-bent nematic phase (Ntb) was studied based on cyanobiphenyl21–26 and structurally related calamitic dimers.27–30 Bent-core mesogenic dimers, which have also been broadly investigated, are usually composed of two bent units,31–38 and in particular, non-symmetric materials39–51 with a bent and a rod-like mesogen have attracted attention as they are a borderline material between bent and rod-like systems. In addition, non-symmetric dimers of bent-core mesogens with discotic52–54 and even dendritic systems55–57 have been reported.
The structure of liquid crystalline dimers consists of two mesogenic units that share a more or less flexible spacer. The first dimers composed of bent-core monomers were linked by an oligosiloxane spacer and exhibited SmCPF and SmCPAF phases depending on the number of siloxane units.31 Since then, further symmetrical LC dimers of bent-core mesogens have been synthesised and studied.32–38 It was shown that the type of central unit (mostly resorcinol and 3,4′-dihydroxybiphenyl) and the lateral fluorine substitution38 of the five-ring monomers did not show a significant effect on the mesomorphic properties. On the other hand, in addition to the chemical structure and size of the mesogenic units, the type and length of the inner flexible spacer played a key role on the transition temperature and mesomorphic behaviour of these dimers. For example, SmCPAF phases were observed only in the materials connected by siloxane or carbosilane units.31,33,34 For the dimers with an alkylene spacer, nematic and smectic phases in the dependence on the spacer length34–36 were found, while for longer spacers, the formation of columnar phases37,38 was also observed. Replacing the alkylene moiety with an oligoethyleneglycol unit resulted in a loss of mesomorphic behaviour,34 while the introduction of a diacetylene rigid spacer led to the appearance of a smectic phase.36
Non-symmetrical dimers composed of a bent-core molecule containing resorcinol at the central core and a cyanobiphenyl rod-like unit connected by alkylene spacers of different lengths exhibited the formation of nematic and smectic phases.39,41–44,49 Only in a few cases were columnar44–46 and SmCP phases47 typical of bent-shaped molecules observed. This again proved that the mesomorphic properties are largely dependent on the spacer length.44–46 The role of the rod-like unit46 and ester linkage orientation45,47,51 have also been investigated for such types of dimers.
Herein, we report a series of W-shaped liquid crystalline dimers for the first time composed of two fully developed bent-core monomers, i.e. each of them possesses two long terminal alkyl chains. Two alkyl chains of the monomers were joined together with an isophthalic acid moiety, which introduced the additional inner molecular bend to finalise the overall W-shape of the dimer. The letter W has also been used recently to characterise the molecular shape of five-ring bent-core materials.58,59 In our case, we are dealing with dimers and the W-shape was created from two bent monomers based on naphthalene central cores derived from naphthalene-2,7-diol and 7-hydroxynaphthalene-2-carboxylic acid, respectively. The mesomorphic properties of the monomers and dimers were tuned by the type of polar linking groups (ester, azo, imino) and by the orientation of the ester linking group(s) in the molecular structure.
Results and discussion
Synthesis
The monomers M-1 to M-7 (Fig. 1) possessing a naphthalene central core were prepared by the synthesis of non-symmetrical bent-core mesogens we described previously.60–64 The non-symmetry of the studied monomers results from the introduction of two different terminal chains, i.e. the hydroxydodecyl and dodecyl chains, respectively. The detailed extensive synthetic procedures for the synthesis of all the intermediates and target monomers and dimers and their full characterisation are summarised in the ESI.†A series of dimers D was prepared from the monomers M. With the exception of dimer D-1, all the compounds D-2 to D-7 were obtained by a one-step reaction of the monomers M with isophthaloyl chloride (for details see the ESI†). In addition to the bend introduced by the naphthalene in the monomer units, the presence of the connecting isophthaloyl moiety results in the formation of an inner bend around 120° to complete the W-shape in the molecular structure of the dimers. The molecular structures of all the dimers D-1 to D-7 are presented in Fig. 2. For synthetic reasons associated with the preparation of the targeted dimer D-1 possessing the imino linking group, the nitro substituted four-ring monomer M-1 represents an intermediate, which is unlike the monomers M-2 to M-7, which possess complete arms of the final dimers D. The structural changes for the monomers M and dimers D (linking groups, ester group orientation) are highlighted in red.
Mesomorphic properties of the monomers
DSC studies were performed for all the studied compounds, and the corresponding phase transition temperatures and associated enthalpy changes are shown in Table 1 for the monomers M and in Table 2 for the corresponding dimers D. Phases were identified from the texture observation under a polarising microscope, and X-ray measurements were performed to confirm phase assignment and to establish the structural parameters.| Comp. | Mp [ΔH] | Tcr [ΔH] | M2 | Ttr [ΔH] | M1 | Ttr [ΔH] | Iso |
|---|---|---|---|---|---|---|---|
| M-1 | 145 [+37.9] | 118 [−31.0] | — | — | N | 127 [−0.4] | • |
| M-2 | 165 [+53.0] | 157 [−42.5] | — | — | — | — | • |
| M-3 | 128 [+17.4] | 117 [−10.6] | — | — | B1Rev | 132 [−5.6] | • |
| M-4 | 170 [+50.9] | 160 [−50.8] | — | — | N | 169 [−1.5] | • |
| M-5 | 152 [+30.0] | 138 [−26.8] | — | — | B1Rev | 172 [−13.1] | • |
| M-6 | 155 [+43.9] | 151 [−46.2] | — | — | N | 154 [−0.4] | • |
| M-7 | 139 [+23.0] | 121 [−31.3] | B1Rev | 162 [−4.0] | SmA | 170 [−4.3] | • |
| Comp. | Mp [ΔH] | Tcr [ΔH] | M4 | Ttr [ΔH] | M3 | Ttr [ΔH] | M2 | Ttr [ΔH] | M1 | Ttr [ΔH] | Iso |
|---|---|---|---|---|---|---|---|---|---|---|---|
| D-1 | 112 [+22.7] | 114 [−14.5] | — | — | — | — | — | — | — | — | • |
| D-2 | 149 [+70.0] | 140 [−39.9] | — | — | — | — | B1Rev | 153 [−17.6] | N | 163 [−1.2] | • |
| D-3 | 133 [+36.6] | 117 [−28.6] | — | — | — | — | B1Rev | 152 [−22.9] | N | 156 [−1.1] | • |
| D-4 | 156 [+64.1] | 133 [−30.1] | — | — | — | — | B1Rev | 149 [−24.5] | N | 162 [−1.3] | • |
| D-5 | 146 [+82.8] | 99 [−28.0] | CrX | 148 [−20.9] | B1Rev | 178 [−2.8] | SmC | 183 [−0.1] | SmA | 192 [−13.3] | • |
| D-6 | 159 [+38.6] | 132 [−32.8] | — | — | B1Rev | 145 [−6.3] | SmC | 156 [−2.6] | N | 171 [−1.2] | • |
| D-7 | 110 [+18.5] | 80 [−10.4] | CrX | 101 [−16.7] | B1Rev | 178 [−3.3] | SmC | 188 [−0.1] | SmA | 191 [−14.1] | • |
DSC plots for selected monomers M-3 and M-6 taken on the second heating and cooling runs are demonstrated in Fig. 3. The four-ring compound M-1 showed a monotropic nematic phase (N). Among the five-ring compounds derived from naphthalene-2,7-diol (M-2, M-3), only M-3 with the ester unit in the outer arm, M-3, formed a liquid crystalline phase, and an enantiotropic columnar B1Rev phase was also observed. Compound M-2 possessing an azo linkage was not mesogenic. The molecular structures of compounds M-4 to M-7 were similar to M-3, but they had a reversed orientation of one (M-4, M-6) or two ester linking groups (M-5, M-7) in the molecular arms terminated with hydroxydodecyl or dodecyl terminal chain, respectively, (see Fig. 1). Changing the orientation of only one ester group resulted in destabilisation of the B1Rev phase and a monotropic nematic phase appears for M-4 and M-6. For compounds M-5 and M-7, the B1Rev phase was observed similarly as for M-3, noticeably with an isotropisation temperature higher by ∼40 K. Furthermore, an orthogonal SmA phase appeared above the B1Rev phase for M-7.
The textures observed under polarising microscope are characteristic. The texture of the columnar B1Rev phase of M-3 at T = 120 °C is shown in Fig. 4 and the texture taken in the N phase of M-1 is presented in the ESI file (Fig. S1†). X-ray measurements performed for compounds M confirmed the phase identification and allowed determination of the structural parameters of the studied mesophases. In Table 3 (the upper part, see below), three structural parameters for the monomers M-3, M-5, and M-7 are summarised. In columnar B1Rev phases, the X-ray pattern is different in the small angle region and can be indexed assuming an oblique primitive crystallographic unit cell. The crystallographic parameter a reflects the number of molecules within the cross-section of the block. The crystallographic parameter b is nearly temperature independent and corresponds to the length of the molecule for monomers M-5 and M-7. Only for M-3 is the crystallographic parameter b smaller than the molecular length, so the molecules are probably tilted.
| Comp. | T/°C | a/Å | b/Å | γ/° |
|---|---|---|---|---|
| M-3 | 140 | 27.8 | 36.6 | 105.7 |
| M-5 | 160 | 82.4 | 52.2 | 100.2 |
| M-7 | 165 | d = 54.1 (SmA) | ||
| 150 | 86.3 | 52.4 | 97.3 | |
| D-2 | 145 | 26.4 | 82.3 | 111.7 |
| D-3 | 130 | 25.6 | 73.7 | 91.2 |
| D-4 | 140 | 50.0 | 42.7 | 102.2 |
| D-5 | 150 | 88.9 | 55.4 | 94.2 |
| D-6 | 150 | d = 45.4 (SmC) | ||
| D-7 | 150 | 88.7 | 54.0 | 98.8 |
The X-ray pattern recorded in the smectic SmA phase of M-7 exhibits a series of sharp, commensurate small angle reflections and a diffuse scattering maximum in the wide angle region, which is characteristic for a lamellar structure with liquid-like molecular packing within the layers, so it confirms the identification of the SmA phase. Additionally, the layer spacing, d, calculated from the position of the X-ray peaks for M-7 corresponds to the calculated length of the molecule. When applying an electric field in the SmA phase of M-7, there are weak electro-optical responses and a field-induced change of birefringence can be observed. In free-standing films, a completely black area is observed under polarising microscopy, which provides evidence of the orthogonal character of the SmA phase.
Mesomorphic properties of dimers
The mesomorphic properties of the studied dimers D-1 to D-7 are summarised in Table 2. The DSC plots for dimers D-3, D-4 and D-5 taken on the second cooling are presented in Fig. 5. Generally, when comparing the dimers with the corresponding monomers M-2 to M-7, we could see that the doubling of the monomer molecular structure resulted in an improvement of the mesomorphic behaviour in the dimers, see Tables 1 and 2. The clearing temperatures of the dimers showed an increase by ∼20 K in comparison with the corresponding monomers (see Tables 1 and 2), with the exception of D-4, which showed isotropisation at a lower temperature than the monomer M-4. Additionally, all the mesogenic dimers formed enantiotropic liquid crystalline phases, while only monomers M-3, M-4 and M-7 were not monotropic. | ||
| Fig. 5 DSC plot for the studied dimers (a) D-3, (b) D-4 and (c) D-5. In the inset of (c), the SmA–SmC phase transition is shown in an enlarged view. | ||
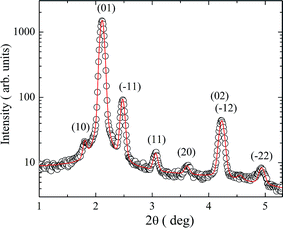
With the exception of D-1, all the dimers exhibited polymorphism, and at least two phases were observed. Dimer D-2 with an azo linkage and D-3 with an ester linkage in the outer arms showed the formation of a narrow nematic and a columnar B1Rev type of mesophase on cooling. In Fig. 6, the textures are presented in the nematic phase at T = 158 °C and the columnar B1Rev phase at T = 150 °C for D-3. Reversion of one ester linkage in the inner arms (dimer D-4) did not exert a substantial effect on the mesomorphic properties and the N–B1Rev sequence was also found. Nevertheless, the structural parameters of the columnar cell were different for D-3 and D-4. In Fig. 7, the X-ray intensity versus the scattering angle is shown for compound D-3 in the B1Rev phase with a fit to a 2D oblique structure. In the inset of Fig. 7, the partially aligned D-3 sample is shown at the same temperature to confirm the indexation and cell determination. The X-ray intensity versus the scattering angle is presented in Fig. 8 for compound D-4 in the B1Rev phase with a fit to a 2D oblique structure. The cell parameters are summarised in Table 3.
 | ||
| Fig. 6 Planar texture of D-3 in (a) the nematic phase at T = 158 °C and (b) the columnar B1Rev at T = 150 °C. | ||
On the other hand, the reversion of both ester groups in the inner arms in D-5 or in the both outer arms in dimer D-7 resulted in a richer polymorphism, and the phase sequence of SmA–SmC–B1Rev–CrX phases was observed in a broad temperature interval. In dimer D-6, in which only one ester unit in the outer arms was reversed in comparison to D-3, the phase sequence N–SmC–B1Rev was proven. Planar samples of D-5 and D-7 preferred the formation of fan-shaped textures. The typical microphotographs of the textures of D-5 are presented in Fig. 9, where one can compare the SmA, SmC, columnar B1Rev and CrX textures. In free-standing films or a one-free-surface sample, a black area was observed under crossed polarisers in the microscope for the SmA phase (see ESI, Fig. S2†). This provides evidence of the orthogonal character of the observed SmA mesophase.
 | ||
| Fig. 9 Planar texture of D-5 in (a) the SmA phase at T = 190 °C, (b) the SmC phase at T = 180 °C, (c) the columnar B1Rev phase at T = 170 °C and (d) the CrX phase at T = 140 °C. | ||
The X-ray patterns of a partially aligned sample of D-5 in the B1Rev phase and in the CrX phase in a broad interval of scattering angles are shown in Fig. 10. In Fig. 10b, one can see that even in the CrX phase, there is no typical crystalline ordering in the wide angle range of the scattering angles. Even below 99 °C, the X-ray patterns do not change, as is typical for the crystallisation. The DSC plot for D-5 (Fig. 5c) shows an opposite peak on the subsequent heating curve, demonstrating an additional crystallisation process. This behaviour reflects that the crystallisation does not exhibit distinct features for D-5. The structural parameters obtained by fitting the X-ray data are summarised in Table 3.
 | ||
| Fig. 10 X-ray patterns of a partially aligned sample of D-5 at (a) T = 155 °C in the B1Rev phase and at (b) T = 120 °C in the CrX phase. | ||
In the columnar mesophase, the structural features for D-5 and for D-7 are very similar. For illustration, we show the X-ray intensity versus the scattering angle for compound D-7 in the B1Rev phase, together with the fit assuming a 2D oblique structure (Fig. 11). Analogous results for compound D-5 are presented in Fig. S3 (ESI†). The most intensive signal of the X-ray pattern was fitted to provide the value of the layer spacing, d. In the B1Rev phase, this value corresponds to the ribbon thickness. The temperature dependences of d for D-5 and D-7 are presented in Fig. S4 and S5,† respectively. Generally, the simultaneous change of both esters in the arms is obviously less important for the mesomorphic and structural properties than the inversion of the esters in any molecular arm.
Schlieren textures in the nematic and the SmC phase and their modification in the B1Rev phase were preferred for the one-free-surface sample. For material D-6, the schlieren textures are depicted in the ESI (Fig. S6†). On the other hand, for the dimers D-5 and D-7, no schlieren is visible in the SmA phase, but a homogeneous dark figure provides evidence of the uniaxial character of this phase. Similarly to the behaviour of the monomers, a field-induced change of birefringence was also observed for D-5 and D-7 in the SmA phase. One can guess that the applied electric field, if high enough, can turn the bent-core molecule along its long molecular axis. Unfortunately, we were not able to detect any polarisation current up to a voltage of 20 V μm−1, and also no dielectric mode was observed in the dielectric spectroscopy in the frequency range of 10 Hz to 1 MHz.
We were not able to detect any polarisation current up to a voltage of 20 V μm−1, and no electro-optical response or dielectric mode was observed in the dielectric spectroscopy in the frequency range of 10 Hz to 1 MHz. This provides evidence that the observed SmA and SmC mesophases exhibit a calamitic character and that there is no polar packing, as can be observed for bent-core compounds.
Discussion
There are only a few studies dealing with the role of the terminal hydroxyl group on the mesomorphic behaviour of rod-like mesogens.65–71 A stabilizing effect of the hydrogen bonding on the formation of the nematic phase,65,68 on the formation of modulated smectic and bilayered smectic phases66,67,69–71 due to the formation of molecular dimers, and on induction of the Ncyb phase69,70 with layer ordering was found. In an example of an amphiphilic bent-shaped material,55 the effect of the diol unit on stabilisation of the smectic mesophase by intermolecular hydrogen bonding was shown. As a consequence, these polar end groups can segregate into distinct sublayers, leading to a bilayered structure. So the preparation of monomers with a terminal hydroxyl group and a comparison with related compounds can shed more light on the role of the terminal hydroxyl group in the bent-core mesogens.First, comparison of the mesomorphic behaviour of the studied monomers M with structurally related derivatives in which both arms were terminated with a dodecyl alkyl chain60–62 showed surprisingly that the naphthalene-based mesogens analogous to M-2 revealed a SmC phase,72 which is contrary to the studied monomer M-2, which was not mesogenic. Compounds corresponding to monomer M-3, which showed only a columnar phase, exhibited a dark-conglomerate smectic phase.60 The nematogenic monomers M-4 and M-6 resemble compounds published previously61 and that exhibited typical SmC phases. Therefore, we can see that for the monomers M, the presence of a terminal hydroxyl group destabilises the mesophase formation and thus they are predominantly monotropic. Additionally, the presence of a terminal hydroxyl group leads to a change in the mesomorphic properties.
We can conclude that the presence of a terminal hydroxyl group does not cause the formation of bi-layers due to the hydrogen bonding among the molecules of monomers and probably does not induce some other special packing of molecules. Nevertheless, due to presence of the nematic phase for M-4 and M-6, one can expect that the hydroxyl group in the terminal position can bring about the dimerisation of monomeric molecules. This can be supported by the fact that the corresponding dimers D-4 and D-6 revealed the nematic phase as well. Derivatives analogous to M-5 and M-7 showed a similar mesomorphic behaviour with the SmA–B1Rev phase sequence.62 In addition, the structural parameters of M-5 and M-7 (Table 3) in the SmA phase corresponded to the same molecular length, and thus one can exclude the dimerisation effect.
Conclusions
Herein, we reported a new type of bent-core liquid crystalline dimers D possessing three bends in their molecular structure, which were derived from bent-core monomers M with a hydroxyl group in the terminal position of one of the alkyl chains. While the presence of the terminal hydroxyl destabilises the mesomorphic properties, all the studied dimers, except D-1, exhibited a rich polymorphism and stable mesomorphic properties. Moreover, with the dimers, we achieved variability of the mesophases and stability in a broad temperature range. Analogous to the monomers, the orientation of the ester linkages influences the mesomorphic behaviour of both the studied monomers and dimers. We can lastly point out that the mesomorphic properties of the studied dimers were more complex in comparison with corresponding monomers.Acknowledgements
This study was supported by projects No. 15-02843S and 16-12150S (Czech Science Foundation), and LD14007 (Ministry of Education, Youth and Sports of the Czech Republic).References
- T. Niori, T. Sekine, J. Watanabe, T. Furukawa and H. Takezoe, J. Mater. Chem., 1996, 6, 1231 RSC.
- R. Amaranatha Reddy and C. Tschierske, J. Mater. Chem., 2006, 16, 907 RSC.
- H. Takezoe and Y. Takanishi, Jpn. J. Appl. Phys., 2006, 45, 597 CrossRef CAS.
- W. Weissflog, H. N. Shreenivasa Murthy, S. Diele and G. Pelzl, Philos. Trans. R. Soc., A, 2006, 364, 2657 CrossRef CAS PubMed.
- W. Weissflog, G. Naumann, B. Košata, M. W. Schröder, A. Eremin, S. Diele, Z. Vakhovskaya, H. Kresse, R. Friedemann, S. Ananda Rama Krishnan and G. Pelzl, J. Mater. Chem., 2005, 15, 4328 RSC.
- J. Szydlowska, J. Mieczkowski, J. Matraszek, D. W. Bruce, E. Gorecka, D. Pociecha and D. Guillon, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2003, 67, 031702 CrossRef CAS PubMed.
- E. Gorecka, N. Vaupotic, D. Pociecha, M. Cepic and J. Mieczkowski, ChemPhysChem, 2005, 6, 1087 CrossRef CAS PubMed.
- E. Gorecka, N. Vaupotic and D. Pociecha, Chem. Mater., 2007, 19, 3027 CrossRef CAS.
- C. T. Imrie and P. A. Henderson, Chem. Soc. Rev., 2007, 36, 2096 RSC.
- C. T. Imrie, P. A. Henderson and G. Y. Yeap, Liq. Cryst., 2009, 36, 755 CrossRef CAS.
- T. Donaldson, P. A. Henderson, M. F. Achard and C. T. Imrie, Liq. Cryst., 2011, 38, 1331 CrossRef CAS.
- T. B. Donaldson, P. A. Henderson, M. F. Achard and C. T. Imrie, J. Mater. Chem., 2011, 21, 10935 RSC.
- J. Watanabe and M. Hayashi, Macromolecules, 1989, 22, 4083 CrossRef CAS.
- H. Ji, K. Q. Zhao, W. H. Yu, B. Q. Wang and P. Hu, Sci. China, Ser. B: Chem., 2009, 52, 975 CrossRef CAS.
- J. Han, Q. Wang, J. Wu and L.-R. Zhu, Liq. Cryst., 2015, 42, 127 CrossRef CAS.
- Y.-K. Kim, R. Breckon, S. Chakraborty, M. Gao, M. S. N. Sprunt, J. T. Gleeson, R. J. Twieg, A. Jakli and O. D. Lavrentovich, Liq. Cryst., 2014, 41, 1345 CrossRef CAS.
- A. K. Prajapati and M. C. Varia, Liq. Cryst., 2013, 40, 1151 CrossRef CAS.
- Y. Shi, X. Wang, J. Wei, H. Yang and J. Guo, Soft Matter, 2013, 9, 10186 RSC.
- M. C. Varia, S. Kumar and A. K. Prajapati, Liq. Cryst., 2012, 39, 933 CrossRef CAS.
- T. Sakurai, K. Tashiro, Y. Honsho, A. Saeki, S. Seki, A. Osuka, A. Muranaka, M. Uchiyama, J. Kim, S. Ha, K. Kato, M. Takata and T. Aida, J. Am. Chem. Soc., 2011, 133, 6537 CrossRef CAS PubMed.
- M. Cestari, S. Diez-Berart, D. A. Dunmur, A. Ferrarini, M. R. de la Fuente, D. J. B. Jackson, D. O. López, G. R. Luckhurst, M. A. Pérez-Jubindo, R. M. Richardson, J. Salud, B. A. Timini and H. Zimermann, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2011, 84, 031704 CrossRef CAS PubMed.
- C. Xingwu, W. Ling, L. Chenyue, X. Jiumei, D. Hangjun, L. Xin, Z. Xiaoguang, H. Wanli and Y. Huai, Chem. Commun., 2013, 49, 10097 RSC.
- J. W. Emsley, M. Lelli, A. Lesage and G. R. Luckhurst, J. Phys. Chem. B, 2013, 117, 6547 CrossRef CAS PubMed.
- Z. Lu, P. A. Henderson, B. J. A. Paterson and C. T. Imrie, Liq. Cryst., 2014, 41, 471 CrossRef CAS.
- A. Hoffmann, A. G. Vanakaras, A. Kohlmeier, G. H. Mehl and D. Photinos, Soft Matter, 2015, 11, 850 RSC.
- Z. Zhang, V. P. Panov, M. Nagaraj, R. J. Mandle, J. W. Goodby, G. R. Luckhurst, J. C. Jones and H. F. Gleeson, J. Mater. Chem. C, 2015, 3, 10007 RSC.
- S. M. Salili, C. Kim, S. Sprunt, J. T. Gleeson, O. Parri and A. Jakli, RSC Adv., 2014, 4, 57419 RSC.
- N. Sebastian, D. O. Lopez, B. Robles-Hernandez, M. R. de la Fuente, J. Salud, M. A. Perez-Jubindo, D. A. Dunmur, G. R. Luckhurst and D. J. B. Jackson, Phys. Chem. Chem. Phys., 2014, 16, 21391 RSC.
- M. G. Tamba, S. M. Salili, C. Zhang, A. Jakli, G. H. Mehl, R. Stannarius and A. Eremin, RSC Adv., 2015, 5, 11207 RSC.
- T. Hamamoto and M. Funahashi, J. Mater. Chem. C, 2015, 3, 6891 RSC.
- G. Dantlgraber, S. Diele and C. Tschierske, Chem. Commun., 2002, 2768 RSC.
- C. Keith, R. Amaranatha Reddy, U. Baumeister, H. Hahn, H. Lang and C. Tschierske, J. Mater. Chem., 2006, 16, 3444 RSC.
- R. Achten, A. Koudijs, M. Giesbers, A. T. M. Marcelis, E. J. R. Sudhölter, M. W. Schroeder and W. Weissflog, Liq. Cryst., 2007, 34, 59 CrossRef CAS.
- B. Kosata, G.-M. Tamba, U. Baumeister, K. Pelz, S. Diele, G. Pelzl, G. Galli, S. Samaritani, E. V. Agina, N. I. Boiko, V. P. Shibaev and W. Weissflog, Chem. Mater., 2006, 18, 691 CrossRef CAS.
- G. Shanker, M. Prehm and C. Tschierske, J. Mater. Chem., 2012, 22, 168 RSC.
- L.-Y. Zhang, Q.-K. Zhang and Y.-D. Zhang, Liq. Cryst., 2013, 40, 1263 CrossRef CAS.
- S. Umadevi and B. K. Sadashiva, Liq. Cryst., 2007, 34, 673 CrossRef CAS.
- S. Umadevi, B. K. Sadashiva, H. N. Shreenivasa Murthy and V. A. Raghunathan, Soft Matter, 2006, 2, 210 RSC.
- N. Sebastián, N. Gimeno, J. Vergara, D. O. López, J. L. Serrano, C. L. Folcia, M. R. de la Fuente and M. B. Ros, J. Mater. Chem. C, 2014, 2, 4027 RSC.
- M.-G. Tamba, A. Bobrovsky, V. Shibaev, G. Pelzl, U. Baumeister and W. Weissflog, Liq. Cryst., 2011, 38, 1531 CrossRef CAS.
- G. Lee, H.-C. Jeong, F. Araoka, K. Ishikawa, J. G. Lee, K.-T. Kang, M. Cepic and H. Takezoe, Liq. Cryst., 2010, 37, 883 CrossRef CAS.
- Y. Wang, H. G. Yoon, H. K. Bisoyi, S. Kumar and Q. Li, J. Mater. Chem., 2012, 22, 20363 RSC.
- C. V. Yelamaggad, S. K. Prasad, G. G. Nair, I. S. Shashikala, D. S. S. Rao, C. V. Lobo and S. Chandrasekhar, Angew. Chem., Int. Ed., 2004, 43, 3429 CrossRef CAS PubMed.
- N. G. Nagaveni, V. Prasad and A. Roy, Liq. Cryst., 2013, 40, 1001 CrossRef CAS.
- M.-G. Tamba, B. Kosata, K. Pelz, S. Diele, G. Pelzl, Z. Vakhovskaya, H. Kresse and W. Weissflog, Soft Matter, 2006, 2, 60 RSC.
- C. V. Yelamaggad, I. S. Shashikala, G. Liao, D. S. S. Rao, S. K. Prasad, Q. Li and A. Jakli, Chem. Mater., 2006, 18, 6100 CrossRef CAS.
- M.-G. Tamba, U. Baumeister, G. Pelzl and W. Weissflog, Liq. Cryst., 2010, 37, 853 CrossRef CAS.
- C. V. Yelamaggad, S. A. Nagamani, S. A. Hiremath and G. G. Nair, Liq. Cryst., 2001, 28, 1009 CrossRef CAS.
- S. K. Prasad, G. G. Nair, D. S. S. Rao, C. V. Lobbo, I. S. Shashikala and C. V. Yelamaggad, Mol. Cryst. Liq. Cryst., 2005, 437, 211 Search PubMed.
- C. V. Yelamaggad, I. S. Shashikala and Q. Li, Chem. Mater., 2007, 19, 6561 CrossRef CAS.
- M. G. Tamba, U. Baumeister, G. Pelzl and W. Weissflog, Ferroelectrics, 2014, 468, 52 CrossRef CAS.
- A. Jakli, I. C. Pintre, J. L. Serrano, M. B. Ros and M. R. de la Fuente, Adv. Mater., 2009, 21, 3784 CrossRef CAS.
- H. K. Bisoyi, H. T. Srinivasa and S. Kumar, Beilstein J. Org. Chem., 2009, 5, 1 Search PubMed.
- J. Vergara, J. Barbera, J. L. Serrano, M. B. Ros, N. Sebastian, R. de la Fuente, D. O. Lopez, G. Fernandez, L. Sanchez and N. Martin, Angew. Chem., Int. Ed., 2011, 50, 12523 CrossRef CAS PubMed.
- D. Kardas, M. Prehm, U. Baumeister, D. Pociecha, R. Amarantha Reddy, G. H. Mehl and C. Tschierske, J. Mater. Chem., 2005, 15, 1722 RSC.
- C. Keith, G. Dantlgraber, R. Amarantha Reddy, U. Baumeister, M. Prehm, H. Hahn, H. Lang and C. Tschierske, J. Mater. Chem., 2007, 17, 3796 RSC.
- N. Gimeno, J. Vergara, M. Cano, J. L. Serrano, M. B. Ros, C. L. Folcia, S. Rodriguez-Conde, G. Sanz-Enguita and J. Etxebarria, Chem. Mater., 2012, 25, 286 CrossRef.
- N. V. S. Rao, M. K. Paul, I. Miyake, Y. Takanishi, K. Ishikawa and H. Takezoe, J. Mater. Chem., 2003, 13, 2880 RSC.
- I. Miyake, Y. Takanishi, N. V. S. Rao, M. K. Paul, K. Ishikawa and H. Takezoe, J. Mater. Chem., 2005, 15, 4688 RSC.
- V. Kozmík, M. Kuchař, J. Svoboda, V. Novotná, M. Glogarová, U. Baumeister, S. Diele and G. Pelzl, Liq. Cryst., 2005, 32, 1151 CrossRef.
- M. Kohout, J. Svoboda, V. Novotná, D. Pociecha, M. Glogarová and E. Gorecka, J. Mater. Chem., 2009, 19, 3153 RSC.
- M. Kohout, J. Svoboda, V. Novotná and D. Pociecha, Liq. Cryst., 2011, 38, 1099 CrossRef CAS.
- A. Kovářová, S. Světlík, V. Kozmík, J. Svoboda, V. Novotná, D. Pociecha, E. Gorecka and N. Podoliak, Beilstein J. Org. Chem., 2014, 10, 794 CrossRef PubMed.
- A. Kovářová, M. Kohout, J. Svoboda and V. Novotná, Liq. Cryst., 2014, 41, 1703 CrossRef.
- A. C. Griffin and S. R. Vaidya, Mol. Cryst. Liq. Cryst., 1989, 173, 85 CrossRef CAS.
- G.-H. Hsiue, L.-H. Wu, C.-J. Hsieh and R.-J. Jeng, Liq. Cryst., 1995, 19, 189 CrossRef CAS.
- D. Pociecha, D. Kardas, E. Gorecka, J. Szydlowska, J. Mieczkowski and D. Guillon, J. Mater. Chem., 2003, 13, 34 RSC.
- S. A. Kuvshinova, D. S. Fokin, V. A. Burmistrov and O. I. Koifman, Russ. J. Org. Chem., 2009, 45, 182 CrossRef CAS.
- A. Yoshizawa, A. Nishizawa, K. Takeuchi, Y. Takanishi and J. Yamamoto, J. Phys. Chem. B, 2010, 114, 13304 CrossRef CAS PubMed.
- W. Nishiya, Y. Takanishi, J. Yamamoto and A. Yoshizawa, J. Mater. Chem. C, 2014, 2, 3677 RSC.
- Y. Kimoto, A. Nishizawa, Y. Takanishi, A. Yoshizawa and J. Yamamoto, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2014, 89, 042503 CrossRef PubMed.
- J. Svoboda, unpublished work.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra06268g |
| This journal is © The Royal Society of Chemistry 2016 |