New D–D–π–A type organic dyes having carbazol-N-yl phenothiazine moiety as a donor (D–D) unit for efficient dye-sensitized solar cells: experimental and theoretical studies†
D. Muenmarta,
N. Prachumraka,
R. Tarsangb,
S. Namungrukc,
S. Jungsuttiwongb,
T. Sudyoadsuka,
P. Pattanasattayavonga and
V. Promarak*a
aDepartment of Material Science and Engineering, School of Molecular Science and Engineering, Vidyasirimedhi Institute of Science and Technology, Wangchan, Rayong, 21210 Thailand. E-mail: vinich.p@vistec.ac.th
bDepartment of Chemistry, Faculty of Science, Ubon Ratchathani University, Warinchumrap, Ubon Ratchathani, 34190 Thailand
cNANOTEC, National Science and Technology Development Agency, 111 Phahonyothin Road, Khlong Luang, Pathum Thani, 12120 Thailand
First published on 7th April 2016
Abstract
A series of novel organic dyes (CPhTnPA, n = 0–2) with the D–D–π–A structural configuration incorporating carbazol-N-yl phenothiazine moiety as a donor (D–D) unit, phenyl oligothiophenes as a π-linker and cyanoacrylic acid as both electron-acceptor and anchoring group were synthesized and characterized for dye sensitized solar cells. Detailed investigation on the relationship between the structure, spectral and electrochemical properties, and performance of DSSC was described here. Time dependent density functional theory (TDDFT) calculations have been performed on the dyes, and the results showed that both electron donors (D–D) can contribute to electron injection upon photo-excitation, either directly or indirectly by internal conversion to the lowest excited state. Dye-sensitized solar cells (DSSCs) using dyes as the sensitizers exhibited good efficiencies. In virtue of co-sensitization, the CPhT2PA based solar cell can achieve photovoltaic efficiency as high as 7.78% (JSC = 15.22 mA cm−2, VOC = 0.74 V and FF = 0.69) which reached 95% with respect to that of the reference N719-based device (8.20%) in parallel investigations.
Introduction
Dye-sensitized solar cells (DSSC) have received extensive attention in the past few decades owing to their potential of low cost production and use as an alternative to conventional silicon based solar cells since their discovery by O'Regan and Grätzel et al. in 1991.1 The dye sensitizer plays an essential role in the performance of DSSC, both in power conversion efficiency (η) as well as device stability. Up to now, DSSCs with a certified η values record of excess 11% have been achieved with ruthenium complexes2 and porphyrin3 dyes. These dyes, however, are expensive and difficult to prepare in high yields. During the past decade, remarkable research has been focused on organic dyes, commonly made with donor–π–acceptor (D–π–A) configuration.4 Interest in these compounds is inspired by an expected lower cost and a by a greater flexibility for the structural control of the energy levels and light-harvesting properties.5 So far, many kinds of organic dyes have been synthesized and tested for effectiveness in DSSCs.6 Only a few examples have shown efficiencies over 10%,7 therefore, optimization of the chemical structures of organic dyes still require further development. Most of the highly efficient organic dyes have a long π-linkage between the donor and acceptor.8 However, the incorporation of long π-conjugated linkages yields extended rod-like molecules, which may ease the recombination of electrons with I−/I3− and increase aggregation between molecules on the TiO2 surface.9 On the basis of such findings, recently, organic dyes with a D–D–π–A structure, where two D moieties are directly linked, were investigated.10 These studies suggested that the co-donor moieties (D–D) not only contributes to the enhancement of the electron-donating ability, but the bulkiness of a co-donor system also hinders the ability of the electrolyte to reach the semiconductor surface and also inhibits dye aggregation, bringing about better performance than the traditional D–π–A structure.11 In this work, we report three novel organic dyes based on a D–D–π–A system, bearing 3-(3,6-di-tert-butylcarbazol-N-yl)-N-octylphenothiazine as the donor unit (D–D), namely CPhTnPA (n = 0–2) (Fig. 1). In this design, phenothiazine as a primary donor, carbazole as an auxiliary donor, phenyl oligothiophenes as a π-linkage, a cyanoacetic acid as an acceptor/anchoring group, respectively, are assembled. Phenothiazine has been successfully used as a donor of many organic dyes12 for DSSC due to its electron-rich nitrogen and sulfur heteroatoms in a heterocyclic structure and high electron-donating ability. Its nonplanar, bent conformation can also rightly impede molecular aggregation and the formation of intermolecular excimers.13 Phenothiazine-based dyes containing variable auxiliary chromophores have been investigated. Auxiliary donors including alkoxyphenyl-,14 1,1,2-triarylethene,13,15 fluorene,16 triarylamine,17 carbazole,18 phenothiazine19 and N-phenylbenzimidazole20 have been substituted on phenothiazine rings to improve the convey of electron from donor to acceptor which offered reasonable results in DSSC. In our case we expected that incorporating an electron donating 3,6-di-tert-butlycarbazole as an auxiliary donor to the phenothiazine donor forming a D–D structure would enhance the ICT process of the dye and lead to dye molecules with high molar extinction coefficient and wide absorption for efficient DSSCs. Moreover, the linear N-ocyl substituent on the phenothiazine and bulky tert-butyl substituents on the carbazole will be also valuable in suppressing electron recombination between electrons injected on the TiO2 surface and I3− ions in the electrolyte and preventing dye aggregation on the TiO2 surface.21 The theoretical and experimental investigations of the properties of these newly synthesized D–D–π–A dyes and their DSSCs are also reported and compared with the traditional N719 dye. This new design allowed us, in a few steps and with good yields, to obtain dyes with remarkable device performances, achieving a very promising η value of 7.78% with CPhT2PA.Results and discussion
Materials synthesis
The synthetic approach used to prepare the new carbazol-N-yl phenothiazine containing CPhTnPA (n = 0–2) dyes is outlined in Scheme 1 and the detailed syntheses are described in the Experimental. The compound 3 was synthesized by an Ullmann-type coupling of 3,6-di-tert-butylcarbazole22 with 3,7-dibromo-N-octylphenothiazine (2)23 in toluene catalyzed by CuI, K3PO4 and trans-1,2-diaminocyclohexane. The other bromide intermediates 5 and 7 were prepared from 3 using a combination of Suzuki cross-coupling and bromination reactions in an iterative manner. The former reaction with 2-thiopheneboronic acid in THF/H2O in the presence of Pd(PPh3)4/Na2CO3 produced compounds 4 and 6 in good yields. The latter bromination with NBS in THF selectively introduced a bromo function to the α-position of the terminal thiophene yielding compounds 5 and 7. The corresponding precursor aldehyde derivatives (8–10) were then synthesized in moderate yields from a coupling between the bromo derivatives (3, 5 and 7) and 4-formylphenylboronic acid under Suzuki coupling conditions. Finally, the aldehydes (8–10) were transformed to the corresponding CPhTnPA (n = 0–2) dyes in 68–75% yields by Knoevenagel condensation reaction with cyanoacetic acid in CHCl3 using piperidine as a basic catalyst. The dyes are red in colour and soluble in most organic solvents. The dyes were thoroughly characterized by IR, NMR, and mass spectral methods, and the observed parameters are consistent with the proposed structures.Optical, thermal and electrochemical properties
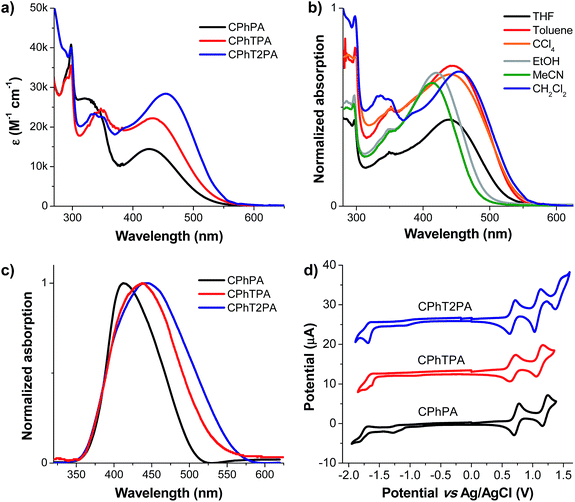
The photophysical properties of the organic dyes CPhTnPA (n = 0–2) were analysed by recording absorption and fluorescence spectra in six different polar solvents, including dichloromethane (CH2Cl2), toluene, tetrahydrofuran (THF), carbon tetrachloride (CCl4), ethanol (EtOH) and acetonitrile (MeCN). The absorption spectra recorded of the dyes in CH2Cl2 solutions shows absorption peaks in the range from 290 to 550 nm (Fig. 2a). The absorption peaks at 298 nm and 320–350 nm relate to the π–π* transition of the carbazole and [carbazol-N-yl phenothiazinyl]oligothiophene conjugated backbone, respectively. The absorption peak above 400 nm corresponds to the intramolecular charge transfer (ICT) from the carbazol-N-yl phenothiazine donor (D–D) unit to the cyanoacrylic acid acceptor unit. This band increasingly red-shifted from 426 (CPhPA) to 432 (CPhTPA) and 455 nm (CPhT2PA), and exhibited enhancement in the molar extinction coefficient (ε) on introduction of additional thiophene units in the conjugation pathway. This probably suggests that in case of CPhPA the charge transfer from the donor unit to cyanoacrylic acid acceptor is weak due to a combination of the poor electron releasing nature of carbazol-N-yl phenothiazine, and the twisted structure and large dihedral angle between the donor and the phenyl linkage. The incorporation of thiophene units in the cases of CPhTPA and CPhT2PA increases the electron-richness of the donor segment due to the delocalization of contributing molecular orbital. Extension of conjugation by oligothiophene units has been found to render a planar bridge between the donor and acceptor and facilitate the donor–acceptor interactions in dipolar compounds.24 In solvents of different polarities, these dyes exhibited a negative solvatochromism in the absorption spectra. As depicted in Fig. 2b, the blue-shift of the ICT band was observed when the polarity of the solvent changes in the order MeCN < EtOH < THF < toluene < CCl4 < CH2Cl2, which is attributed to the effective solvation of the dyes.25 The sign of the solvatochromism depends on the difference in dipole moment between the ground and excited states of the chromophore. A change in the solvent polarity will lead to different stabilization of the ground and excited states, and thus, a change in the energy gap between these electronic states. The significant red-shift observed in chlorinated solvents such as dichloromethane arises due to the rapid relaxation of polarizable electrons in the excited state.26 As depicted in Fig. 2c, the absorption spectra of the dyes anchored on TiO2 film displayed a blue-shift of the ICT-band compared to their spectra in solution, as observed in most organic dyes.27The thermal properties of the dyes investigated by thermogravimetric analysis (TGA) reveal that they are thermally stable compounds, with temperatures at 5% weight loss (T5d) well over 330 °C (Table 1). The high thermal stability of the dye is crucial for the lifetime of the DSSCs.28
| Dye | λsolabs (ε)a (nm, M−1 cm−1) | λfilmabsb (nm) | λsolema (nm) | T5dc (°C) | E1/2 vs. Ag/AgCld (V) | Eoptg/Eeleg/Ecalge (eV) | EHOMO/ELUMOf (eV) | Egapg (eV) |
|---|---|---|---|---|---|---|---|---|
| a Measured in CH2Cl2 solutions.b Measured as dyes adsorbed on TiO2 films.c Obtained from TGA measured at 10 °C min−1 under N2.d Obtained from CV measured in CH2Cl2 solutions at a scan rate of 50 mV s−1 and n-Bu4NPF6 as electrolyte.e Calculated from Eoptg = 1240/λonset; Eeleg = Ereonset − Eoxonset; Ecalg calculated by TD-B3LYP/6-31G(d,p) in CH2Cl2 by C-PCM model.f Estimated from EHOMO = −(4.44 + Eoxonset); ELUMO = Eoptg + EHOMO.g Calculated from Egap = 3.90 (CB of TiO2) − ELUMO. | ||||||||
| CPhPA | 426 (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 404) 404) |
417 | 482 | 330 | −1.75, 0.74, 1.20 | 2.37/2.33/2.34 | −5.11/−2.74 | 1.16 |
| CPhTPA | 432 (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 204) 204) |
431 | 531 | 332 | −1.67, 0.69, 1.11 | 2.35/2.14/2.20 | −5.08/−2.73 | 1.17 |
| CPhT2PA | 455 (28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 491) 491) |
448 | 541 | 344 | −1.68, 0.66, 1.08, 1.43 | 2.29/2.10/2.16 | −5.04/−2.75 | 1.15 |
The electrochemical properties of the dyes were investigated using cyclic and differential pulse voltammetry (CV and DPV) in CH2Cl2 solutions. The energies of the HOMO and LUMO levels of the dyes were deduced from these measurements. From these values, the thermodynamic driving force for electron injection into the conduction band of TiO2 and regeneration feasibility of the oxidized dyes by electrolyte can be evaluated. All dyes displayed one quasi-reversible reduction and multiple quasi-reversible oxidative waves (Fig. 2c, and Table 1). The reduction (−1.75 to −1.67 V) corresponds to the reduction of the acceptor moiety. The first oxidation potentials (0.74–0.66 V) of dyes are a response to the removal of an electron from the phenothiazine donor, while the second oxidation potentials (1.20–1.08 V) originate from the carbazole moiety owing to its weaker electron-releasing ability than phenothiazine.29 A gradual decrease of these oxidation potentials was observed as the number of thiophene unit in the π-linkage increased, as also detected in other oligothiophene derivatives.30 The HOMO–LUMO gaps are plotted in Fig. 3. The HOMO levels (−5.11 to −5.04 eV) of all dyes were more positive than the reducing potential of the iodine/iodide pair (−4.90 eV),31 ensuring sufficient driving force for the oxidized dyes to recapture an electron from the electrolyte. The LUMO levels is important in defining the possibility of an electron transferring from the dye into the conduction band of the TiO2 and can be estimated from the following conversion; ELUMO = EHOMO + Eoptg (Table 1). The energy gap (Egap) between the LUMO level of the dyes and the conduction band edge of the TiO2 (−3.90 eV) should be at least 0.2 eV for efficient electron injection.32 The Egap of CPhTnPA dyes are in the range of 1.15–1.17 eV, thus warranting the viability of electron injection.
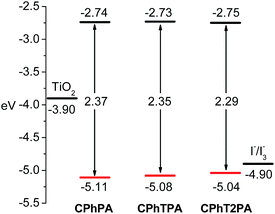 | ||
| Fig. 3 Schematic diagram of the energy levels of TiO2 conduction band, dyes, and I−/I3− redox couple. | ||
Theoretical calculations
The optimized ground state geometries of CPhTnPA dyes are showed in Fig. 4 and S1,† and the selected dihedral angles are listed in Table 2. The dihedral angles formed between carbazole (D1) and phenothiazine (D2) planes in all molecules were as large as ca. 60°, resulting in bulky structures that might help to prevent close π–π aggregation between the dye molecules. Non-coplanar geometry can also reduce contact between molecules and enhance their thermal stability.33 The dihedral angles between the linker bridge and the electron acceptor of all designed dyes (i.e. values of π3–A in Table 2) were found to be less than 1 degree, implying that the π-conjugated benzene bridge was found to be coplanar with cyanoacrylic acid. This coplanarity between the benzene bridge and the electron acceptor would lead to an effective movement of electrons from donor to acceptor after the light absorption through excitation. Similar results were found in the dihedral angles between π1–π2 and π2–π3, which were calculated to be in the range of 11.50–14.55 degree. This suggests that a π-electron from the donor moiety (D1–D2) can effectively delocalize to the acceptor moiety, and thus transfer to the conduction band of TiO2.| Dye | Dihedral angle (°)/intergroup | ||||
|---|---|---|---|---|---|
| D1–D2 | D2–π1 | π1–π2 | π2–π3 | π3–A | |
| a D1 = carbazole; D2 = phenothiazine; π1–2 = thiophene; π3 = benzene; A = cyanoacrylic acid. | |||||
| CPhPA | 59.48 | −31.00 | — | — | 0.49 |
| CPhTPA | 60.80 | −22.55 | 14.55 | — | −0.21 |
| CPhT2PA | 59.98 | −24.03 | 11.50 | −13.56 | 0.33 |
The plotted of electronic structures for HOMO and LUMO of all dyes are shown in Fig. 4. The charge distributions on HOMO are localized on the donor moiety, while the electron density of LUMO is mainly localized on the bridge and acceptor units. These orbital densities show that the distribution of the HOMOs and LUMOs of all the dyes are well-separated, implying that the electron transition from HOMO to LUMO can be regarded as intramolecular charge transfer (ICT), a major characteristics of D–π–A-based dyes, which facilitates π-orbital overlap via the quinoid resonance structure and produces a consequent redshift absorption of the dyes.34 The calculated energy levels of these dyes obtained at the B3LYP/6-31G(d) level of theory are depicted in Fig. 4. It can be clearly seen that increasing the number of thiophene units in the π-linkage significantly affects the HOMO and LUMO levels of all dyes. The HOMO levels of these dyes systematically increases when increasing the number of thiophene units. Moreover, adding the carbazole auxiliary donor into the phenothiazine primary donor forming D–D moiety makes the HOMOs of the dyes closer to the redox potential of the electrolyte system, which confirms the capability of dye regeneration from its oxidized state. As a result, the calculated HOMO–LUMO gaps (EH–L) are narrowed in order of 2.33 ≈ 2.38 > 2.29 eV for CPhPA, CPhTPA and CPhT2PA dyes, respectively.
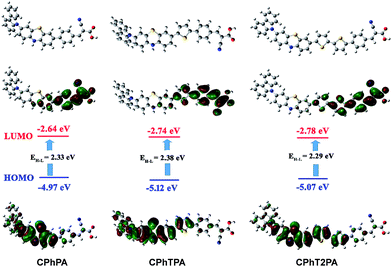 | ||
| Fig. 4 Optimized structures (top), HOMO (middle) and LUMO (bottom) of the dyes and their energies calculated by B3LYP/6-31G(d,p) in CH2Cl2 solvent. | ||
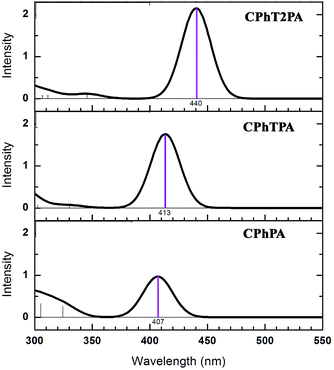
The simulated visible absorption spectra of the new designed carbazole–phenothiazine-based dyes are shown in Fig. 5, the spectrum for each dyes is consistent with an ICT character. The calculated wavelength and oscillator strength together with main transition characters are listed in Table S1.† As can be seen from Fig. 5, the number of conjugation length is increased from 0 to 2 units with an increase in conjugation length of the dyes. The calculated maximum absorption spectra (λmax) are in order of 407, 413 and 440 nm for CPhPA, CPhTPA and CPhT2PA dyes, respectively. This indicates that the dye CPhT2PA which is the broadest of absorption wavelength shows the highest light harvesting ability. Also, the calculated absorption intensities (molar extinction coefficient; ε) of CPhPA, CPhTPA and CPhT2PA dyes are increased in order of 8.6 × 10−4, 12.4 × 10−4, 15.2 × 10−4 M−1 cm−1, respectively. This agrees well with the electronic structure analysis discussed above.
The adsorption energy (Eads) of all dyes on TiO2 is calculated to be about −23 kcal mol−1, indicating the strong interactions between the dyes and the TiO2 cluster (Fig. 6a and Table 3). The vertical transition dipole moment (μ⊥, Debye) of CPhTnPA dyes are calculated for a better understanding of the performance of the intramolecular charge transfer upon photoexcitation (Table 3). For increasing of n from 0 to 2, the μ⊥ values give the trend as CPhPA (6.78 D) < CPhTPA (10.12 D) < CPhT2PA (10.95 D). For a better understanding of the performance of the intramolecular charge transfer upon photoexcitation, the μ⊥ values of dye–TiO2 complexes were calculated and compared with those values of the free dyes (Table 3). The μ⊥ values of dye–TiO2 complexes (μ⊥ = 34.91–48.00 D) are significantly higher than those of the free dyes, ensuring the feasibility of electron flow from the dyes into the TiO2. For increasing of n from 0 to 2, the μ⊥ values give the trend as CPhPA–TiO2 (34.91 D) < CPhTPA–TiO2 (40.89D) < CPhT2PA–TiO2 (48.00 D). To simulate the light harvesting phenomenon on the prototype device system, we also examined the charge density difference between the ground and the excited state of CPhPA–TiO2 to describe the direction of charge transfer upon the electron excitation, see Fig. 6b. It is clearly shown that charge has transferred from carbazole–phenothiazine donor of the dye to TiO2 substrate throughout the whole dye-backbone. This indicates a strong ICT character with electron direct injection mechanism which is beneficial when dye is harvesting light in the device system. This result indicates that the new designed carbazole–phenothiazine-based dyes are suitable to use for efficient dyes with high ICT character. Based on ICT properties, energy level, UV-Vis absorption spectra and μ⊥ values the dye CPh2TPA is predicted to give the best DSC performance among these dyes, these results excellently agree with the highest observed JSC of CPhT2PA-based solar cell (12.87 mA cm−1) compared to others, however, the observed VOC value is still relatively low.
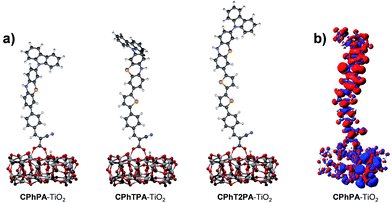 | ||
| Fig. 6 (a) Optimized structures of the dye adsorbed onto TiO2 cluster and (b) density difference between ground and excited state calculated by TD CAM-B3LYP/6-31G(d) showing strong ICT character. | ||
| Complex | Eads (kcal mol−1) | Dipole moment (μ⊥, D) |
|---|---|---|
| a μ⊥ = the dipole moment in the perpendicular direction to the TiO2 surface. | ||
| Free dye | ||
| CPhPA | — | 6.78 |
| CPhTPA | — | 10.12 |
| CPhT2PA | — | 10.95 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Dye–TiO2 | ||
| CPhPA–TiO2 | −23.5 | 34.91 |
| CPhTPA–TiO2 | −23.3 | 40.89 |
| CPhT2PA–TiO2 | −23.4 | 48.00 |
Photovoltaic properties
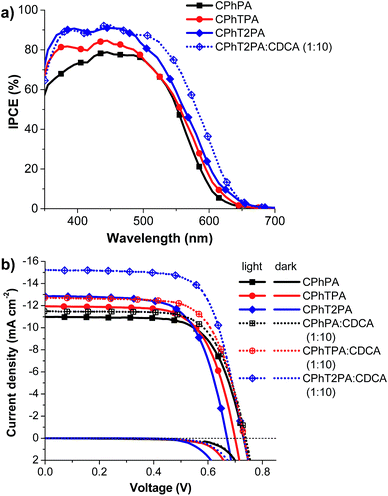
DSSCs based on the CPhTnPA dyes were fabricated by coating them on the surface of TiO2 as sensitizers. The incident photo-to-current conversion efficiencies (IPCE) and J–V characteristics of all dyes measured under simulated AM 1.5 solar irradiation (100 mW cm−2) are presented in Fig. 8. The short-circuit photocurrent density (JSC), open-circuit photovoltage (VOC), fill factor (FF), and power conversion efficiency (η) are summarized in Table 4, where N719 was used as a reference dye. As depicted in Fig. 7a, the CPhTPA-based device exhibits lower than 80% IPCEs in the wavelength range from 350 to 650 nm, whereas the noticeably enhanced IPCEs (>80%) of the CPhTPA- and CPhT2PA-based cells in the whole visible region (360–500 nm) clearly represent the importance of introduction of thiophene unit in the π-linkage. The maximum IPCE value for DSSC based on the CPhT2PA dye reaches 90% in the wavelength range from 380 to 450 nm and is much higher than that for CPhPA and CPhTPA, which is in good agreement with their absorption spectra (Fig. 2a). The IPCE spectrum for DSSC based on CPhT2PA is the broadest among all dyes. These observations explain the higher JSC exhibited by CPhT2PA dye (12.87 mA cm−2). As is well known, increasing and broadening the IPCE spectra is preferred for larger photocurrent JSC.35| Device | Dye | Dye adsorbeda (molecule per cm2) | JSC (mA cm−2) | VOC (V) | FF | η (%) | RCT (ohm) at bias −0.70 V | τ (ms) at bias −0.70 V |
|---|---|---|---|---|---|---|---|---|
| a Measured by desorption method.30 | ||||||||
| I | CPhPA | 6.58 × 1016 | 10.98 | 0.73 | 0.68 | 5.51 | 76.90 | 31.35 |
| II | CPhTPA | 7.13 × 1016 | 11.92 | 0.70 | 0.68 | 5.67 | 35.81 | 16.90 |
| III | CPhT2PA | 7.43 × 1016 | 12.87 | 0.67 | 0.68 | 5.86 | 21.69 | 14.50 |
| IV | CPhPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CDCA (1 CDCA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) 10) |
6.42 × 1016 | 11.46 | 0.74 | 0.69 | 5.88 | 110.29 | 40.88 |
| V | CPhTPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CDCA (1 CDCA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) 10) |
6.80 × 1016 | 12.69 | 0.73 | 0.68 | 6.33 | 63.61 | 24.63 |
| VI | CPhT2PA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CDCA (1 CDCA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) 5) |
7.41 × 1016 | 13.10 | 0.70 | 0.68 | 6.21 | — | — |
| VII | CPhT2PA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CDCA (1 CDCA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) 10) |
6.67 × 1016 | 15.22 | 0.74 | 0.69 | 7.78 | 92.37 | 27.12 |
| VIII | CPhT2PA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CDCA (1 CDCA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) 20) |
4.95 × 1016 | 13.29 | 0.74 | 0.68 | 6.68 | — | — |
| IX | N719 | 5.83 × 1016 | 16.81 | 0.73 | 0.69 | 8.20 | — | — |
As depicted in Fig. 7b, the JSC values of the DCCSs are in the order of CPhT2PA (12.87 mA cm−2) > CPhTPA (11.92 mA cm−2) > CPhPA (10.98 mA cm−2), which are consistent with their IPCE characteristics. However, when we consider VOC values of all devices, it is found that the VOC of the DSSCs are in the opposite tendency to the JSC, and in the order of CPhPA (0.73 V) > CPhTPA (0.70 V) > CPhT2PA (0.67 V). Field factor (FF) of these DSSCs are identical with the value of 0.68. On the co-effect of JSC and VOC, the η values of the DSSCs of CPhT2PA, CPhTPA and CPhPA dyes are 5.86, 5.67 and 5.51%, respectively. The increasing in the JSC values of CPhTnPA-based DSSCs with an increasing the number of thiophene units in the π-linkage of the dyes is due to the π-conjugated extension in the molecules, resulting in a greater redshift of the absorption maxima and higher absorption intensity (ε). It has been known that JSC value of a DSSC is strongly associated with the light harvesting efficiency (LHE) of the dye. In general, the LHE is related to the ε value of the dye.36 Hence, a higher molar extinction coefficient yields a higher LHE and JSC. The long π-conjugated extension in the dye molecule, on the other hand, induces the dye aggregations on the TiO2 surface. As listed in Table 4, the optimum dye adsorption amounts on the TiO2 films (molecule per cm) of the DSSCs measured by the desorption method37 are in the order of CPhT2PA (7.43 × 1016) > CPhTPA (7.13 × 1016) > CPhPA (6.58 × 1016). Accordingly, the DSSC fabricated with CPhT2PA and CPhTPA dyes as sensitizer will experience a greater tendency to form π–π-stacked dye aggregates on the TiO2 surface than CPhPA. It has been known that dye aggregation can directly affect photoelectron injection efficiency of the dye and thereby the overall conversion efficiency.38 It has been also reported that the VOC values are decreased with extending π-conjugated chain length of the dyes.39
In order to further understand the behaviour of the CPhTnPA dyes on the TiO2 surface, experiments using co-absorption with chenodeoxycholic acid (CDCA) were carried out. CDCA has been demonstrated to suppress dye aggregation and effectively improve solar cell performance.40 Upon the addition of 10 mM CDCA to the dye solution as a co-adsorbent to inhibit aggregation, much improved photovoltaic performances (JSC, VOC and η) were realized (Table 4). For CPhT2PA and CPhTPA dyes, the VOC values of DSSCs in the presence of CDCA increased because of effective suppression of the dark current. Fixing other conditions, the effect of CDCA content in the CPhT2PA dye solution on the DSSC performance was investigated (Table 4). The JSC increased until a dye/CDCA molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and then decreased with further increasing CDCA amount. Conversely, the VOC improved at increasing contents of CDCA in the dye solution. As a consequence, the highest η value of 7.78% (JSC = 15.22 mA cm−2, VOC = 0.74 V, FF = 0.69) can be obtained at dye/CDCA molar ratio of 1
10 and then decreased with further increasing CDCA amount. Conversely, the VOC improved at increasing contents of CDCA in the dye solution. As a consequence, the highest η value of 7.78% (JSC = 15.22 mA cm−2, VOC = 0.74 V, FF = 0.69) can be obtained at dye/CDCA molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 which notably increases by 32.7% compared with the pure dye solution sensitized TiO2 electrode (5.86%). The efficiency of CPhT2PA dye reached >95% of the efficiency of devices made with the standard N719 complex (η = 8.20%, JSC = 16.81 mA cm−2, VOC = 0.73 V, FF = 0.69), under the same conditions. The outstanding improvement of η is ascribed mostly to the break-up of dye aggregation which benefits from the appropriate amount of the dye adsorbed on the TiO2 electrode (dye/CDCA molar ratio of 1
10 which notably increases by 32.7% compared with the pure dye solution sensitized TiO2 electrode (5.86%). The efficiency of CPhT2PA dye reached >95% of the efficiency of devices made with the standard N719 complex (η = 8.20%, JSC = 16.81 mA cm−2, VOC = 0.73 V, FF = 0.69), under the same conditions. The outstanding improvement of η is ascribed mostly to the break-up of dye aggregation which benefits from the appropriate amount of the dye adsorbed on the TiO2 electrode (dye/CDCA molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). Too much amount of CDCA (dye/CDCA molar ratio of 1
10). Too much amount of CDCA (dye/CDCA molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) reduces the absorbed amount of the dye on TiO2 films and the ability to harvest light of the dye, and thus results in low JSC.41 On the other hand, too low amount of CDCA (dye/CDCA molar ratio of 1
20) reduces the absorbed amount of the dye on TiO2 films and the ability to harvest light of the dye, and thus results in low JSC.41 On the other hand, too low amount of CDCA (dye/CDCA molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) partly maintains aggregation of the dyes on the TiO2 electrode leading to inefficient electron injection and lower η. Under the optimum CDCA doping content (dye/CDCA molar ratio of 1
5) partly maintains aggregation of the dyes on the TiO2 electrode leading to inefficient electron injection and lower η. Under the optimum CDCA doping content (dye/CDCA molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), CPhTPA and CPhPA dyes yield an increasing the photovoltaic performances from 5.67 to 6.33% and 5.51 to 5.58%, respectively. The result suggests that a new CPhT2PA dye having carbazol-N-yl phenothiazine moiety as a donor (D–D) unit is a promising alternative to the traditional metal–organic dyes used in DSSCs.
10), CPhTPA and CPhPA dyes yield an increasing the photovoltaic performances from 5.67 to 6.33% and 5.51 to 5.58%, respectively. The result suggests that a new CPhT2PA dye having carbazol-N-yl phenothiazine moiety as a donor (D–D) unit is a promising alternative to the traditional metal–organic dyes used in DSSCs.
Electrochemical impedance spectroscopy
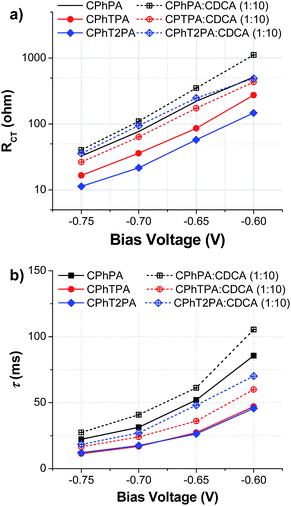
The charge recombination at the TiO2/electrolyte interface was studied by electrochemical impedance spectroscopy (EIS), which is a useful steady-state method to elucidate the electronic and ionic processes occurring in DSSCs. Fig. 8 shows the EIS Nyquist plots measured under dark with a forward bias of −0.70 V and equivalent circuit model for the DSSCs. Clearly two semicircles were visible for all the devices. The larger semicircle in the Nyquist plots is attributed to the charge-transfer resistance (RCT) at the TiO2 electrode/dye/electrolyte interface. The value of RCT can be related to the charge-recombination rate between the injected electron and electron acceptor I3− in the electrolyte. The RCT values can be calculated by fitting the curves using an equivalent circuit model, and are plotted in Fig. 9a and listed in Table 4.42 The fitted RCT values (at −0.7 V) of the CPhTnPA-based DSSCs are in the sequence CPhPA (76.90 ohm) > CPhTPA (35.81 ohm) > CPhT2PA (21.69 ohm). A larger RCT value signifies the slower rate of charge recombination or smaller charge recombination at the TiO2/electrolyte interface of DSSC, ensuing in a higher VOC. Accordingly, these observations are in agreement with the VOC tendency observed: CPhPA > CPhTPA > CPhT2PA. The RCT values of the DSSCs of all dyes co-adsorption with CDCA are higher than that of DSSCs based on the corresponding free dye (Fig. 9a), indicating that the charge recombination of the device sensitized by dye/CDAC is efficiently suppressed, leading to the improvement of the VOC, the photocurrent and photovoltaic of the DSSCs. Especially, in the case of CPhT2PA/CDCA-based cell its RCT value (92.37 ohm) is nearly 4 times higher than that of CPhT2PA-based cell (21.69 ohm), agreeing with a huge improve of its VOC from 0.67 to 0.73 V. Moreover, the differences in VOC of these DSSCs can also be elucidated by the effective electron lifetime. The electron lifetime (τ) values of the DSSCs extracted from the angular frequency (ωmin) at the mid-frequency peak in the Bode phase plot using τ = 1/ωmin,43 and are plotted in Fig. 9b and listed in Table 4. The τ values (at −0.65 V) of the CPhTnPA-based DSSCs follow the trend CPhPA (31.35 ms) > CPhTPA (16.90 ms) > CPhT2PA (14.50 ms). As depicted in Fig. 9b, the dyes/CDCA-based cells exhibit a higher τ values or longer electron lifetime compared with the DSSCs based on the corresponding free dye, leading to lower rate of charge recombination, and thus, improved VOC. These results are in a very good agreement with the trend of their VOC. Accordingly, the enhancement of solar cell performance by co-adsorption with CDCA are well explained by the EIS results.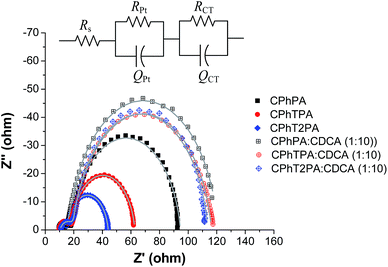 | ||
| Fig. 8 Nyquist plots measured under a forward bias of −0.70 V and equivalent circuit model for the DSSCs. | ||
Experimental
Materials and methods
All chemicals were purchased from Aldrich, Acros, or Thai companies and used as received. THF and CH2Cl2 were purified and dried using standard protocols. Compounds 144 and 223 were synthesized by using the reported procedure.1H and 13C NMR spectra were performed with an AVANCE 500 MHz spectrometer. FTIR spectra were recorded with a RXI spectrometer. UV/Vis spectra were measured in CH2Cl2 with a UV Lambda 25 spectrometer. Diffuse reflectance spectra of dye adsorbed on TiO2 were measured at room temperature with a UV-3101 spectrophotometer using BaSO4 as a standard. The measured reflectance spectra were then converted into absorption spectra by the Kubelka–Munk method. Thermogravimetric analysis (TGA) was performed with a TG-DTA 8120 thermal analyzer with heating rate of 10 °C min−1 under a N2 flow. CV measurements were performed with a PGSTAT 12 potentiostat equipped with a platinum counter electrode, glassy carbon working electrode, and Ag/AgCl reference electrode in CH2Cl2 under an Ar flow with n-Bu4NPF6 as a supporting electrolyte at a scan rate of 50 mV s−1. Melting points were measured with an IA 9100 series digital melting-point instrument and are uncorrected. High-resolution MALDI-TOF mass spectra were recorded with an Autoflex II TOF/TOF mass spectrometer.
All of calculations were carried out using GAUSSIAN 09.45 The ground state geometries were fully optimized using DFT at the B3LYP level with the 6-31G(d,p) basis set in CH2Cl2. The dye–(TiO2)38 systems were fully optimized by the Perdew–Burke–Ernzerhof (PBE) functional with the double numerical polarization (DNP) basis set. The core electron was treated with DFT-semicore pseudopotentials (DSPPs) by DMol3 code in Materials Studio 5.5™.
Synthesis of 3
A mixture of 2 (13.02 g, 27.74 mmol), 3,6-di-tert-butylcarbazole (2.30 g, 8.25 mmol), CuI (0.88 g, 4.62 mmol), K3PO4 (4.91 g, 23.12 mmol) and trans-1,2-diaminocyclohexane (0.57 g, 4.99 mmol) in toluene (80 ml) was stirred at reflux under N2 for 24 h. After cooling water (100 ml) was added, the mixture was extracted with CH2Cl2 (100 ml × 3). The combined organic phase was washed with water (100 ml), brine solution (100 ml), dried over anhydrous Na2SO4, filtered and evaporated to dryness. The residue was subjected to column chromatography over silica gel eluting with a mixture of CH2Cl2 and hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) to obtain a white solid (4.16 g, 75%). 1H NMR (500 MHz, acetone-D6) δ 0.94 (3H, d, J = 7.0 Hz), 1.37–1.47 (10H, m), 1.53 (18H, s), 1.58–1.65 (2H, m), 4.14 (2H, t, J = 7.0 Hz), 7.14 (1H, d, J = 9.0 Hz), 7.36 (3H, t, J = 8.5 Hz), 7.43 (1H, d, J = 2.0 Hz), 7.46 (2H, dd, J = 2.0 Hz and 8.5 Hz), 7.51 (1H, dd, J = 2.5 Hz and 6.0 Hz), 7.57 (2H, dd, J = 2.0 Hz, J = 6.5 Hz), 8.36 (1H, d, J = 1.5 Hz) ppm; 13C NMR (125 MHz, acetone-D6) δ 13.58, 22.41, 26.55, 26.58, 31.54, 34.43, 47.33, 54.96, 109.10, 114.28, 116.30, 116.73, 117.39, 123.29, 123.60, 125.34, 126.07, 129.33, 130.26, 132.83, 139.39, 142.65, 144.07 ppm; MALDI-TOF (m/z) (M+) calcd for C40H47BrN2S: 666.2643, found 666.3814.
3) to obtain a white solid (4.16 g, 75%). 1H NMR (500 MHz, acetone-D6) δ 0.94 (3H, d, J = 7.0 Hz), 1.37–1.47 (10H, m), 1.53 (18H, s), 1.58–1.65 (2H, m), 4.14 (2H, t, J = 7.0 Hz), 7.14 (1H, d, J = 9.0 Hz), 7.36 (3H, t, J = 8.5 Hz), 7.43 (1H, d, J = 2.0 Hz), 7.46 (2H, dd, J = 2.0 Hz and 8.5 Hz), 7.51 (1H, dd, J = 2.5 Hz and 6.0 Hz), 7.57 (2H, dd, J = 2.0 Hz, J = 6.5 Hz), 8.36 (1H, d, J = 1.5 Hz) ppm; 13C NMR (125 MHz, acetone-D6) δ 13.58, 22.41, 26.55, 26.58, 31.54, 34.43, 47.33, 54.96, 109.10, 114.28, 116.30, 116.73, 117.39, 123.29, 123.60, 125.34, 126.07, 129.33, 130.26, 132.83, 139.39, 142.65, 144.07 ppm; MALDI-TOF (m/z) (M+) calcd for C40H47BrN2S: 666.2643, found 666.3814.
Synthesis of 4 and 6
A mixture of 3 and 5 (3.28 mmol), 2-thiopheneboronic acid (3.52 mmol), Pd(PPh3)4 (0.07 mmol), 2 M Na2CO3 (15 ml) in THF (40 ml) was degassed with N2 for 5 min. The mixture was stirred at reflux under N2 for 24 h. After cooling, the mixture was extracted with CH2Cl2 (70 ml × 2). The combined organic phase was washed with water (70 ml), brine solution (70 ml), dried over anhydrous Na2SO4, filtered and evaporated to dryness. The residue was subjected to column chromatography over silica gel eluting with a mixture of CH2Cl2 and hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) to obtain
3) to obtain
4 as yellow solids (75%). FTIR (KBr) ν 3412, 2956, 2917, 2857, 2357, 1604, 1476, 1327, 1294, 1262, 1198, 875 cm−1; 1H NMR (500 MHz, acetone-D6) δ 0.96 (3H, t, J = 7.0 Hz), 1.38 (7H, t, J = 12.5 Hz), 1.53 (20H, s), 1.63 (2H, t, J = 7.5 Hz), 4.17 (2H, t, J = 7.0 Hz), 7.18 (1H, t, J = 4.5 Hz), 7.22 (1H, d, J = 8.5 Hz), 7.38 (3H, d, J = 8.5 Hz), 7.46–7.51 (4H, m), 7.56–7.62 (4H, m), 8.36 (2H, s) ppm; 13C NMR (125 MHz, acetone-D6) δ 13.45, 22.38, 26.52, 26.63, 31.42, 31.57, 34.11, 47.23, 109.11, 116.28, 116.38, 116.63, 122.71, 123.31, 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 362, 124.11, 124.76, 125.10, 125.31, 126.01, 132.63, 139.47, 142.65, 144.14 ppm; MALDI-TOF (m/z) (M+) calcd for C44H50N2S2: 670.3415, found 670.5211.
362, 124.11, 124.76, 125.10, 125.31, 126.01, 132.63, 139.47, 142.65, 144.14 ppm; MALDI-TOF (m/z) (M+) calcd for C44H50N2S2: 670.3415, found 670.5211.
6 as yellow solids (87%). FTIR (KBr) ν 3549, 3412, 2956, 2846, 2362, 1635, 1618, 1467, 1481, 1448, 1360, 1294, 1259, 809 cm−1; 1H NMR (500 MHz, acetone-D6) δ 0.87 (3H, d, J = 6.5 Hz), 1.30 (7H, s), 1.45 (18H, s), 1.57 (2H, d, J = 7.0 Hz), 4.10 (2H, t, J = 7.0 Hz), 7.11 (1H, t, J = 3.6 Hz), 7.15 (1H, d, J = 8.5 Hz), 7.26 (1H, d, J = 3.5 Hz), 7.31 (3H, d, J = 8.5 Hz), 7.38 (2H, d, J = 4.5 Hz), 7.43 (2H, d, J = 5.5 Hz), 7.52 (3H, m), 8.30 (2H, s) ppm; 13C NMR (125 MHz, acetone-D6) δ 13.49, 22.40, 26.53, 26.62, 31.43, 31.59, 34.39, 47.23, 109.12, 116.31, 116.40, 116.67, 123.31, 123.56, 123.64, 123.69, 123.84, 124.76, 124.82, 124.87, 125.31, 125.45, 126.02, 128.10, 128.73, 132.68, 139.43, 142.64, 144.01 ppm; MALDI-TOF (m/z) (M+) calcd for C48H52N2S3: 752.3293, found 752.7253.
Synthesis of 5 and 7
To a solution of 4 and 6 (0.31 mmol) in THF (20 ml) was added with NBS (0.32 mmol) in small portions over 10 min. The mixture was stirred at room temperature for 1 h. Water (5 ml) was added and the mixture was extracted with CH2Cl2 (50 ml × 2). The combined organic phase was washed with water (50 ml), brine solution (50 ml), dried over anhydrous Na2SO4, filtered and evaporated to dryness. The residue was subjected to a recrystallization with a mixture of CH2Cl2 and methanol to yield5 as yellow solid (86%). FTIR (KBr) ν 3401, 2956, 2923, 2857, 2357, 1476, 1294, 1292, 877 cm−1; 1H NMR (500 MHz, acetone-D6) δ 0.86 (3H, t, J = 7.0 Hz), 1.28–1.40 (9H, m), 1.44 (18H, s), 1.53 (2H, m), 4.05 (2H, t, J = 7.0 Hz), 7.08 (1H, d, J = 8.5 Hz), 7.12 (1H, d, J = 4.0 Hz), 7.20 (1H, d, J = 4.0 Hz), 7.28 (3H, t, J = 8.5 Hz), 7.34 (1H, d, J = 2.0 Hz), 7.39–7.44 (3H, m), 7.47 (2H, dd, J = 2.0 Hz, J = 8.7 Hz), 8.26 (2H, s) ppm; 13C NMR (125 MHz, acetone-D6) δ 13.54, 22.42, 26.55, 26.61, 31.49, 31.60, 34.42, 47.26, 109.12, 116.27, 116.36, 116.65, 123.14, 123.29, 123.62, 123.92, 124.93, 125.29, 125.42, 125.99, 128.22, 131.41, 132.71, 139.40, 142.63, 143.92, 144.77 ppm; MALDI-TOF (m/z) (M+) calcd for C44H49BrN2S2: 748.2521, found 748.455.
7 as yellow solids (98%). FTIR (KBr) ν 3549, 3483, 3412, 2956, 2923, 2851, 2362, 1637, 1613, 1478, 1454, 1259, 787 cm−1; 1H NMR (500 MHz, acetone-D6) δ 0.94 (3H, t, J = 7.0 Hz), 1.36–1.48 (10H, m), 1.52 (18H, s), 1.60 (2H, m), 4.13 (2H, t, J = 6.5 Hz), 7.11 (2H, d, J = 4.0 Hz), 7.15 (1H, d, J = 3.5 Hz), 7.24 (1H, d, J = 4.0 Hz), 7.28 (3H, t, J = 7.0 Hz), 7.36 (2H, s), 7.40 (1H, d, J = 8.5 Hz), 7.51 (4H, t, J = 7.6 Hz), 8.30 (2H, s) ppm; 13C NMR (125 MHz, acetone-D6) δ 13.49, 22.40, 26.53, 26.61, 31.44, 31.59, 34.39, 47.23, 109.12, 116.28, 116.40, 116.66, 123.31, 123.63, 124.06, 124.86, 124.95, 125.29, 125.42, 125.99, 128.46, 131.39, 132.70, 132.70, 134.42, 139.40, 142.56, 142.64, 143.92, 144.66 ppm; MALDI-TOF (m/z) (M+) calcd for C48H51BrN2S3: 830.2398, found 830.3154.
Synthesis of aldehydes 8, 9 and 10
A mixture of 3, 5 and 7 (0.75 mmol) 4-formylphenylboronic acid (0.80 mmol), Pd(PPh3)4 (0.02 mmol), 2 M Na2CO3 (8 ml) in THF (15 ml) was degassed with N2 for 5 min. The mixture was stirred at reflux under N2 for 24 h. After cooling, the mixture was extracted with CH2Cl2 (50 ml × 2). The combined organic phase was washed with water (50 ml), brine solution (50 ml), dried over anhydrous Na2SO4, filtered and evaporated to dryness. The residue was subjected to column chromatography over silica gel eluting with a mixture of CH2Cl2 and hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to obtain
2) to obtain
8 as orange solids (60%). FTIR (KBr) ν 3554, 3473, 3406, 2956, 2928, 2851, 1700, 1602, 1264, 1168, 809 cm−1; 1H NMR (500 MHz, acetone-D6) δ 0.87 (3H, d, J = 6.5 Hz), 1.30–1.41 (10H, m), 1.45 (18H, s), 1.56 (2H, d, J = 7.0 Hz), 4.10 (2H, t, J = 6.5 Hz), 7.20 (1H, d, J = 8.5 Hz), 7.31 (3H, t, J = 8.5 Hz), 7.37 (1H, s), 7.42 (1H, d, J = 8.5 Hz), 7.50 (2H, d, J = 8.5 Hz), 7.61 (1H, s), 7.65 (1H, d, J = 8.5 Hz), 7.89 (2H, d, J = 8.0 Hz), 7.99 (2H, d, J = 8.0 Hz), 8.31 (2H, s), 10.08 (1H, s) ppm; 13C NMR (125 MHz, acetone-D6) δ 13.49, 22.40, 26.54, 26.62, 31.15, 31.44, 31.59, 34.39, 47.23, 109.12, 116.32, 116.41, 116.71, 123.31, 123.63, 124.80, 125.30, 125.61, 125.76, 126.00, 126.63, 126.75, 130.06, 132.74, 133.86, 135.40, 139.41, 142.65, 143.93, 145.26, 145.36 and 191.72 ppm; MALDI-TOF (m/z) (M+) calcd for C47H52N2OS: 692.3800, found 693.5470.
9 as orange solids (60%). FTIR (KBr) ν 3549, 3462, 3412, 2950, 2923, 2857, 1695, 1559, 1476, 1443, 1256, 872 cm−1; 1H NMR (500 MHz, acetone-D6) δ 0.94 (3H, t, J = 7.0 Hz), 1.37 (10H, m), 1.52 (18H, s), 1.62 (2H, d, J = 7.5 Hz), 4.16 (2H, t, J = 7.0 Hz), 7.19 (1H, d, J = 8.5 Hz), 7.34–7.39 (3H, m), 7.44 (1H, d, J = 2.5 Hz), 7.48 (7H, dd, J = 2.5 Hz, J = 8.5 Hz), 7.55–7.59 (3H, m), 7.64 (1H, d, J = 8.5 Hz), 7.70 (1H, d, J = 4.0 Hz), 7.95 (2H, d, J = 8.0 Hz), 8.02 (2H, d, J = 8.0 Hz), 8.31 (2H, s), 10.10 (1H, s) ppm; 13C NMR (125 MHz, acetone-D6) δ 14.23, 22.43, 26.60, 26.67, 31.55, 31.61, 47.39, 109.12, 116.21, 116.30, 116.61, 123.29, 123.60, 124.09, 125.31, 125.47, 125.96, 126.62, 128.61, 130.29, 12.74, 135.41, 139.42, 142.64, 191.04 ppm; MALDI-TOF (m/z) (M+) calcd for C51H54N2OS2: 774.3678, found 774.5890.
10 as orange solids (24%). FTIR (KBr) ν 3549, 3412, 2956, 2857, 1695, 1599, 1481, 1262, 872 cm−1; 1H NMR (300 MHz, acetone-D6) δ 0.96 (3H, t, J = 7.0 Hz), 1.38–1.53 (10H, m), 1.55 (18H, s), 1.64 (2H, d, J = 7.5 Hz), 4.18 (2H, t, J = 7.0 Hz), 7.25 (1H, d, J = 8.5 Hz), 7.38 (4H, dd, J = 6.0 Hz, J = 8.5 Hz), 7.45–7.48 (3H, m), 7.51 (2H, dd, J = 3.5 Hz, J = 10.0 Hz), 7.58 (3H, dd, J = 2.0 Hz, J = 10.5 Hz), 7.65 (1H, dd, J = 2.0 Hz, J = 8.5 Hz), 7.75 (1H, d, J = 4.0 Hz), 7.99 (2H, d, J = 8.0 Hz), 8.06 (2H, d, J = 8.0 Hz), 8.37 (1H, d, J = 1.5 Hz), 10.135 (1H, s) ppm; 13C NMR (125 MHz, acetone-D6) δ 13.46, 22.38, 26.52, 26.63, 31.42, 31.58, 47.28, 109.11, 116.33, 116.39, 116.71, 123.33, 123.63, 123.79, 123.92, 124.88, 124.98, 125.05, 125.32, 125.44, 125.58, 126.05, 126.61, 128.56, 130.31, 132.75, 135.19, 139.26, 142.67, 143.96, 144.71, 191.13 ppm; MALDI-TOF (m/z) (M+) calcd for C55H56N2OS3: 856.3555, found 856.7830.
Synthesis of CPhTnPA (n = 0–2)
A mixture of 8, 9 and 10 (0.34 mmol) and cyanoacetic acid (0.51 mmol) in CHCl3 (25 ml) was added with piperidine (1.02 mmol). The mixture was stirred at reflux for overnight. The solvent was in vacuo to dryness. Purification by column chromatography over silica gel eluting with a mixture of CH2Cl2 and hexane (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) followed by recrystallization with a mixture of CH2Cl2 and methanol gave
1) followed by recrystallization with a mixture of CH2Cl2 and methanol gave
CPhPA as red solids (73%). mp > 250 °C; FTIR (KBr) ν 3547, 3474, 3418, 2956, 2926, 2857, 2215, 1617, 1593, 1477, 1363, 1260, 1187, 809 cm−1; 1H NMR (500 MHz, acetone-D6) δ 0.94 (3H, t, J = 7.0 Hz), 1.36–1.52 (28H, m), 1.62 (2H, t, J = 7.5 Hz), 4.16 (2H, t, 6.5 Hz), 7.29 (1H, d, J = 8.5 Hz), 7.39 (3H, t, J = 8.5 Hz), 7.46 (1H, s), 7.49 (1H, d, J = 8.5 Hz), 7.57 (2H, dd, J = 1.5 Hz, J = 9.0 Hz), 7.72 (1H, s), 7.77 (1H, d, J = 7.5 Hz), 7.96 (2H, d, J = 8.0 Hz), 8.21 (2H, d, J = 7.5 Hz), 8.37 (2H, s) ppm; 13C NMR (125 MHz, acetone-D6) δ 13.72, 22.42, 26.57, 26.68, 31.59, 31.65, 34.53, 47.29, 109.24, 116.57, 116.90, 123.29, 123.76, 124.61, 125.33, 125.50, 125.62, 126.61, 126.73, 131.16, 131.37, 132.66, 133.69, 139.42, 142.71, 143.93, 145.25 ppm; MALDI-TOF (m/z) (M+) calcd for C50H53N3O2S: 759.3858, found 759.5574.
CPhTPA as red solids (58%). mp > 250 °C; FTIR (KBr) ν 3551, 3474, 3409, 2952, 2918, 2862, 2215, 1638, 1613, 1477, 1336, 1260, 798 cm−1; 1H NMR (500 MHz, acetone-D6) δ ppm; 0.95 (3H, s), 1.37 (10H, s), 1.52 (18H, s), 1.63 (2H, s), 4.16 (2H, d, J = 6.5 Hz), 7.23 (1H, d, J = 8.5 Hz), 7.38 (4H, dd, J = 2.5 Hz, J = 8.5 Hz), 7.48–7.51 (2H, m), 7.57 (3H, d, J = 4.0 Hz), 7.63–7.70 (3H, m), 7.89 (1H, s), 8.07 (1H, s), 8.37 (2H, s) ppm; 13C NMR (125 MHz, acetone-D6) δ 13.65, 22.41, 26.55, 26.66, 31.60, 34.49, 47.28, 109.21, 116.43, 116.52, 116.81, 123.29, 123.72, 123.96, 124.67, 125.12, 125.32, 125.38, 126.07, 128.76, 132.63, 139.41, 142.68, 143.50, 143.93, 144.57 ppm; MALDI-TOF (m/z) (M+) calcd for C54H55N3O2S2: 841.3736, found 841.4650.
CPhT2PA as red solids (71%). mp > 250 °C; FTIR (KBr) ν 3556, 3478, 3405, 2956, 2926, 2215, 1617, 1593, 1477, 1363, 1260, 1187, 809 cm−1; 1H NMR (500 MHz, acetone-D6) δ 0.95–1.33 (3H, m), 1.37–1.54 (28H, m), 1.64 (2H, t, J = 7.0 Hz), 4.15 (2H, d, J = 6.5 Hz), 7.24 (1H, d, J = 8.5 Hz), 7.38 (3H, d, J = 8.5 Hz), 7.47 (3H, s), 7.51 (2H, d, J = 8.5 Hz), 7.58–7.61 (3H, m), 7.64 (1H, d, J = 8.5 Hz), 7.76 (1H, d, J = 7.0 Hz), 7.97 (2H, d, J = 8.5 Hz), 8.20 (2H, d, J = 8.5 Hz), 8.37 (2H, s) ppm; 13C NMR (125 MHz, acetone-D6) δ 13.57, 22.41, 26.54, 26.65, 31.54, 31.59, 47.29, 109.18, 116.43, 116.49, 116.66, 116.81, 123.31, 123.70, 123.94, 124.79, 125.23, 125.62, 126.09, 126.64, 128.55, 131.40, 132.69, 135.23, 137.53, 138.28, 139.43, 141.16, 142.69, 143.95, 144.65 ppm; MALDI-TOF (m/z) (M+) calcd for C58H57N3O2S3: 923.3613, found 923.6046.
Fabrication and characterisations of DSSC devices
Fluorine-doped SnO2 (FTO) conducting glasses (8 W sq.−1, TEC8, Pilkington) were used for transparent conducting electrodes. In the first step, FTO glasses (1.3 × 2 cm2) were treated by spin-coating of blocking layer (BL-1, Dye Sol Ltd) at 3500 rpm, 20 s and then heated at 450 °C for 30 min and then cooled down to room temperature. Next step, the double nanostructured thick films of TiO2 pastes (∼9 + 5 μm thickness) that consisted of a transparent (DSL 18NR-T, Dye Sol Ltd.) double-coated on the FTO and scattering (WER2-O, Dye Sol Ltd.) also double-coated were screen-printed on the treated FTO glasses with cell geometry of 0.5 × 0.5 cm2, and heated to 500 °C for 30 min, and then cooled to 50 °C. The thickness of the TiO2 film was controlled by selection of screen mesh size and repetition of printing. The warm TiO2 electrodes (50 °C) coated on FTO glasses were immersed in the dye or dyes/CDCA solutions (0.3 mM in EtOH) in the dark at room temperature for 24 h to stain the dye onto the TiO2 surfaces. Excess amounts of dye were removed by rinsing with EtOH. The Pt counter electrode was prepared on a predrilled 8 W sq.−1, TEC8, FTO glass (Pilkington) by means of the thermal decomposition of 4 mM H2PtCl6 in a mix solvent of acetyl lactone and 2-methoxyethanol at 450 °C and then cooled down to room temperature. The dye-adsorbed TiO2 photoanode and Pt counter electrode were assembled into a sealed cell by heating a Dye Sol film (30 μm thickness) gasket as a spacer between the electrodes. An electrolyte solution that comprised 0.03 M I2, 0.05 M LiI, 0.6 M tetrapropylammonium iodide (TPAI), and 0.5 M 4-tert-butypyridine (TBP) in a mixed solvent of acetonitrile and valeronitrile (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 v/v) was filled through the predrilled hole by a vacuum backfilling method. The hole was capped by using hot-melt sealing film (Dye Sol film, 30 μm thickness) and a thin glass cover. Finally, copper conducting tape (Scotch 3M) and silver paint (SPI supplies) were coated on the electrodes to enhance the electric contact. The J–V of the DSSCs was measured by using a Keithley 2400 source meter unit in a 4-terminal sense configuration. The data were averaged from forward and backward scans with a bias step and a delay time of 10 mV and 40 ms, respectively, according to the method of Koide and Han.46 The simulating sunlight was provides by Newport sun simulator 96000 equipped with an AM 1.5G filter. To minimize the error of measurements, the irradiation intensity of 100 mW cm−2 was approximated with a calibrated BS-520 Si photodiode (Bunnkoukeiki Co., Ltd., Japan), which its spectral response was very similar to that of the DSSCs. The spectral output of the lamp was also matched to the standard AM 1.5G solar spectrum in region of 350–750 nm by the aid of KG-5 filter with spectral mismatch less than 2% as reported by Ito et al.47 Incident photon to electron conversion efficiency (IPCE) of the device under short-circuit condition were performed by mean of an Oriel 150 W Xe lamp fitted with a Cornerstone TM 130 1/8 m monochromator as a monochromatic light source, a Newport 818-UV silicon photodiode as power density calibration and a Keithley 6485 picoammeter. All measurements were performed using a black plastic mask with an aperture area of 0.25 cm2 and no mismatch correction for the efficiency conversion data. Electrochemical impedance spectra (EIS) were analysed using EA163 eDAQ potentiostat integrated with ERZ100 eDAQ Z100 electrochemical impedance analyzer at bias potential of −0.65 V in dark condition. Nyquist plots of all DSSCs were recorded over a frequency range of 50 mHz to 100 kHz with amplitude of 10 mV and fitted using ZMAN software (WonTech Co. Ltd.) and equivalent circuit Rs–RPt∥QPt–RCT∥QCT. The Rs denotes the ohmic series resistance of the cell, RPt stands for charge resistance at Pt/electrolyte electrode and RCT represents the charge recombination resistance at the TiO2/electrolyte interface. The Q parameters are the constant phase elements.
15 v/v) was filled through the predrilled hole by a vacuum backfilling method. The hole was capped by using hot-melt sealing film (Dye Sol film, 30 μm thickness) and a thin glass cover. Finally, copper conducting tape (Scotch 3M) and silver paint (SPI supplies) were coated on the electrodes to enhance the electric contact. The J–V of the DSSCs was measured by using a Keithley 2400 source meter unit in a 4-terminal sense configuration. The data were averaged from forward and backward scans with a bias step and a delay time of 10 mV and 40 ms, respectively, according to the method of Koide and Han.46 The simulating sunlight was provides by Newport sun simulator 96000 equipped with an AM 1.5G filter. To minimize the error of measurements, the irradiation intensity of 100 mW cm−2 was approximated with a calibrated BS-520 Si photodiode (Bunnkoukeiki Co., Ltd., Japan), which its spectral response was very similar to that of the DSSCs. The spectral output of the lamp was also matched to the standard AM 1.5G solar spectrum in region of 350–750 nm by the aid of KG-5 filter with spectral mismatch less than 2% as reported by Ito et al.47 Incident photon to electron conversion efficiency (IPCE) of the device under short-circuit condition were performed by mean of an Oriel 150 W Xe lamp fitted with a Cornerstone TM 130 1/8 m monochromator as a monochromatic light source, a Newport 818-UV silicon photodiode as power density calibration and a Keithley 6485 picoammeter. All measurements were performed using a black plastic mask with an aperture area of 0.25 cm2 and no mismatch correction for the efficiency conversion data. Electrochemical impedance spectra (EIS) were analysed using EA163 eDAQ potentiostat integrated with ERZ100 eDAQ Z100 electrochemical impedance analyzer at bias potential of −0.65 V in dark condition. Nyquist plots of all DSSCs were recorded over a frequency range of 50 mHz to 100 kHz with amplitude of 10 mV and fitted using ZMAN software (WonTech Co. Ltd.) and equivalent circuit Rs–RPt∥QPt–RCT∥QCT. The Rs denotes the ohmic series resistance of the cell, RPt stands for charge resistance at Pt/electrolyte electrode and RCT represents the charge recombination resistance at the TiO2/electrolyte interface. The Q parameters are the constant phase elements.
Conclusions
In summary, we have synthesized new D–D–π–A type organic dyes, CPhTnPA (n = 0–2) comprising of 3-(3,6-di-tert-butylcarbazol-N-yl)-N-octylphenothiazine as a donor moiety (D–D), phenyl oligothiophenes as a π-linkage, a cyanoacetic acid as an acceptor/anchoring group, and the compounds are fully characterized. The experimental and theoretical results indicated that the combination of phenothiazine as a primary donor and carbazole as an auxiliary donor enhanced the donor ability of the dye, resulting in a wide and more intense absorption spectra that consequently caused an enlargement in the photocurrent JSC of the DSSC. Under simulated AM 1.5 irradiation (100 mW cm−2), the devices constructed with these dyes exhibited η values of CPhPA (5.51%), CPhTPA (5.67%) and CPhT2PA (5.86%). The addition of CDCA as a co-adsorbent improved the cell performances (JSC, VOC and η) of the DSSCs. With the assistance of co-absorption, the CPhT2PA-based DSSC with a maximum η of 7.78% (JSC = 15.22 mA cm−2, VOC = 0.74 V, FF = 0.69) was achieved. The high energy conversion efficiency of CPhT2PA sensitized cells indicates that the 3-(3,6-di-tert-butylcarbazol-N-yl)-N-octylphenothiazine as a donor unit (D–D) could be a promising candidate for application in highly efficient D–D–π–A organic sensitizers.Acknowledgements
This work was supported by the Thailand Research Fund (TRF) (Grant No. DBG5580001).Notes and references
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef.
- M. Grätzel, J. Photochem. Photobiol., A, 2004, 164, 3 CrossRef CAS; A. Yella, H.-W. Lee, H. N. Tsao, C. Yi, A. K. Chandiran, M. K. Nazeeruddin, E. W.-G. Diau, C.-Y. Yeh, S. M. Zakeeruddin and M. Grätzel, Science, 2011, 334, 629 CrossRef PubMed.
- S. Mathew, A. Yella, P. Gao, R. Humphry-Baker, B. F. E. Curchod, N. Ashari-Astani, I. Tavernelli, U. Rothlisberger, Md. K. Nazeeruddin and M. Grätzel, Nat. Chem., 2014, 6, 242 CrossRef CAS PubMed; M. Urbani, M. Grätzel, M. K. Nazeeruddin and T. Torres, Chem. Rev., 2014, 114, 12330 CrossRef PubMed.
- B.-G. Kim, K. Chung and J. Kim, Chem.–Eur. J., 2013, 19, 5220 CrossRef CAS PubMed; M. Liang and J. Chen, Chem. Soc. Rev., 2013, 42, 3453 RSC; Y. Ooyama and Y. Harima, ChemPhysChem, 2012, 13, 4032 CrossRef PubMed.
- C.-P. Lee, R. Y.-Y. Lin, L.-Y. Lin, C.-T. Li, T.-C. Chu, S.-S. Sun, J. T. Lin and K.-C. Ho, RSC Adv., 2015, 5, 23810 RSC; Y.-S. Yen, H.-H. Chou, Y.-C. Chen, C.-Y. Hsu and J. T. Lin, J. Mater. Chem., 2012, 22, 8734 RSC.
- M.-D. Damaceanu, M. Mihaila, C.-P. Constantin, S. Chisca, B.-C. Serban, C. Diaconu, O. Buiu, M. Pavelescu and M. Kusko, RSC Adv., 2015, 5, 53687 RSC; F. Guo, X. Liu, Y. Ding, F. Kong, W. Chen, L. Zhou and S. Dai, RSC Adv., 2016, 6, 13433 RSC; N. Shibayama, Y. Inoue, M. Abe, S. Kajiyama, H. Ozawa, H. Miura and H. Arakawa, Chem. Commun., 2015, 51, 12795 RSC; D. Demeter, P. Melchiore, P. Biagini, S. Jungsuttiwong, R. Po and J. Roncali, J. Mater. Chem. C, 2015, 3, 7756 RSC; P. Thongkasee, A. Thangthong, N. Janthasing, T. Sudyoadsuk, S. Namuangruk, T. Kaewin, S. Jungsuttiwong and V. Promarak, ACS Appl. Mater. Interfaces, 2014, 6, 8212 Search PubMed; T. Mori, H. Saomoto, K. Machitani, K. Inoue, Y. Aoki, T. Koshitani, N. Koumura and T. N. Murakami, RSC Adv., 2016, 6, 13964 RSC.
- Z. Yao, M. Zhang, H. Wu, L. Yang, R. Li and P. Wang, J. Am. Chem. Soc., 2015, 137, 3799 CrossRef CAS PubMed; M. Zhang, Y. Wang, M. Xu, W. Ma, R. Li and P. Wang, Energy Environ. Sci., 2013, 6, 2944 Search PubMed; K. Kakiage, Y. Aoyama, T. Yano, T. Otsuka, T. Kyomen, M. Unno and M. Hanaya, Chem. Commun., 2014, 50, 6379 RSC; W. Zeng, Y. Cao, Y. Bai, Y. Wang, Y. Shi, M. Zhang, F. Wang, C. Pan and P. Wang, Chem. Mater., 2010, 22, 1915 CrossRef.
- S. Ahmad, E. Guillen, L. Kavan, M. Grätzel and Md. K. Nazeeruddin, Energy Environ. Sci., 2013, 6, 3439 Search PubMed; A. Mishra, M. K. R. Fischer and P. Bauerle, Angew. Chem., Int. Ed., 2009, 48, 2474 CrossRef CAS PubMed.
- Z. J. Ning, Y. Fu and H. Tian, Energy Environ. Sci., 2010, 3, 1170 CAS.
- P. Dai, L. Yang, M. Liang, H. Dong, P. Wang, C. Zhang, Z. Sun and S. Xue, ACS Appl. Mater. Interfaces, 2015, 7, 22436 Search PubMed; T. Sudyoadsuk, S. Pansay, S. Morada, R. Rattanawan, S. Namuangruk, T. Kaewin, S. Jungsuttiwong and V. Promarak, Eur. J. Org. Chem., 2013, 23, 5051 CrossRef CAS; S. Namuangruk, R. Fukuda, M. Ehara, J. Meeprasert, T. Khanasa, S. Morada, T. Kaewin, S. Jungsuttiwong, T. Sudyoadsuk and V. Promarak, J. Phys. Chem. C, 2012, 116, 25653 Search PubMed; Z. Yao, M. Zhang, R. Li, L. Yang, Y. Qiao and P. Wang, Angew. Chem., Int. Ed., 2015, 54, 5994 CrossRef PubMed; M.-D. Zhang, H.-X. Xie, X.-H. Ju, L. Qin, Q.-X. Yang, H.-G. Zheng and X.-F. Zhou, Phys. Chem. Chem. Phys., 2013, 15, 634 RSC; A. Baheti, P. Singh, C.-P. Lee, K. R. J. Thomas and K.-C. Ho, J. Org. Chem., 2011, 76, 4910 CrossRef PubMed.
- Y. Wu and W. Zhu, Chem. Soc. Rev., 2013, 42, 2039 RSC.
- M. Marszalek, S. Nagane, A. Ichake, R. Humphry-Baker, V. Paul, S. M. Zakeeruddin and M. Grätzel, J. Mater. Chem., 2012, 22, 889 RSC; H. N. Tian, X. C. Yang, R. K. Chen, A. Hagfeldt and L. C. Sun, Energy Environ. Sci., 2009, 2, 674 Search PubMed; Z. B. Xie, A. Midya, K. P. Loh, S. Adams, D. J. Blackwood, J. Wang, X. J. Zhang and Z. k. Chen, Prog. Photovoltaics, 2010, 18, 573 Search PubMed; Y. Hua, S. Chang, J. He, C. Zhang, J. Zhao, T. Chen, W.-Y. Wong, W.-K. Wong and X. Zhu, Chem.–Eur. J., 2014, 20, 6300 CrossRef CAS PubMed; G. Marotta, M. A. Reddy, S. P. Singh, A. Islam, L. Han, F. D. Angelis, M. Pastore and M. Chandrasekharam, ACS Appl. Mater. Interfaces, 2013, 5, 9635 Search PubMed.
- H. N. Tian, X. C. Yang, R. K. Chen, Y. Z. Pan, L. Li, A. Hagfeldt and L. C. Sun, Chem. Commun., 2007, 43, 3741 RSC; W. J. Wu, J. B. Yang, J. L. Hua, J. Tang, L. Zhang, Y. T. Long and H. Tian, J. Mater. Chem., 2010, 20, 1772 RSC.
- Y. Hua, S. Chang, D. Huang, X. Zhou, X. Zhu, J. Zhao, T. Chen, W.-Y. Wong and W.-K. Wong, Chem. Mater., 2013, 25, 2146 CrossRef CAS; Z. Iqbal, W.-Q. Wu, H. Zhang, P.-L. Hua, X. Fang, D.-B. Kuang, L. Wang, H. Meier and D. Cao, Dyes Pigm., 2013, 99, 299 CrossRef; Z. Iqbal, W.-Q. Wu, H. Zhang, P.-L. Hua, X. Fang, D.-B. Kuang, L. Wang, H. Meier and D. Cao, Dyes Pigm., 2013, 96, 722 CrossRef; C. Chen, X. Yang, M. Cheng, F. Zhang, J. Zhao and L. Sun, ACS Appl. Mater. Interfaces, 2013, 5, 10960 Search PubMed; M. Cheng, X. Yang, F. Zhang, J. Zhao and L. Sun, J. Phys. Chem. C, 2013, 117, 9076 Search PubMed.
- C. Chen, J.-Y. Liao, Z. Chi, B. Xu, X. Zhang, D.-B. Kuang, Y. Zhang, S. Liu and J. Xu, J. Mater. Chem., 2012, 22, 8994 RSC.
- Y. Hua, S. Chang, H. Wang, D. Huang, J. Zhao, T. Chen, W.-Y. Wong, W.-K. Wong and X. Zhu, J. Power Sources, 2013, 243, 253 CrossRef CAS.
- C.-J. Yang, Y. J. Chang, M. Watanabe, Y.-S. Hon and T. J. Chow, J. Mater. Chem., 2012, 22, 4040 RSC.
- T. Meyer, D. Ogermann, A. Pankrath, K. Kleinermanns and T. J. J. Müller, J. Org. Chem., 2012, 77, 3704 CrossRef CAS PubMed.
- Y. J. Chang, P.-T. Chou, Y.-Z. Lin, M. Watanabe, C.-J. Yang, T.-M. Chin and T. J. Chow, J. Mater. Chem., 2012, 22, 21704 RSC.
- G. B. Bodedla, K. R. J. Thomas, C.-T. Li and K.-C. Ho, RSC Adv., 2014, 4, 53588 RSC.
- T. Khanasa, N. Jantasing, S. Morada, N. Leesakul, R. Tarsang, S. Namuangruk, T. Kaewin, S. Jungsuttiwong, T. Sudyoadsuk and V. Promarak, Eur. J. Org. Chem., 2013, 13, 2608 CrossRef.
- P. Moonsin, N. Prachumrak, R. Rattanawan, T. Keawin, S. Jungsuttiwong, T. Sudyoadsuk and V. Promarak, Chem. Commun., 2012, 48, 3382 RSC.
- I. Lachaise, C. Jakubowicz, R. Barhdadi and M. Troupel, New J. Chem., 1997, 21, 1015 CAS.
- K. R. J. Thomas, Y.-C. Hsu, J. T. Lin, K.-M. Lee, K.-C. Ho, C.-H. Lai, Y.-M. Cheng and P.-T. Chou, Chem. Mater., 2008, 20, 1830 CrossRef CAS; A. Mishra, N. Pootrakulchote, M. Wang, S.-J. Moon, S. M. Zakeeruddin, M. Grätzel and P. A. Bäuerle, Adv. Funct. Mater., 2011, 21, 963 CrossRef.
- Z.-S. Wang, F.-Y. Li and C.-H. Huang, J. Phys. Chem. B, 2001, 105, 9210 CrossRef CAS; P. Singh, A. Baheti and K. R. J. Thomas, J. Org. Chem., 2011, 76, 6134 CrossRef PubMed.
- A. Granzhan, H. Ihmels and G. Viola, J. Am. Chem. Soc., 2007, 129, 1254 CrossRef CAS PubMed.
- M. Lu, M. Liang, H.-Y. Han, Z. Sun and S. Xue, J. Phys. Chem. C, 2011, 115, 274 CAS.
- M.-S. Tsai, Y.-C. Hsu, J. T. Lin, H.-C. Chen and C.-P. Hsu, J. Phys. Chem. C, 2007, 111, 18785 CAS.
- X. Q. Zhu, Z. Dai, A. Yu, S. A. Wu and J. P. Cheng, J. Phys. Chem. B, 2008, 112, 11694 CrossRef CAS PubMed; H. Tian, X. Yang, J. Cong, R. Chen, C. Teng, J. Liu, Y. Hao, L. Wang and L. Sun, Dyes Pigm., 2010, 84, 62 CrossRef.
- J. Khunchalee, R. Tarsang, N. Prachumrak, S. Jungsuttiwong, T. Keawin, T. Sudyoadsuk and V. Promarak, Tetrahedron, 2012, 68, 8416 CrossRef CAS.
- L. Yu, K. Fan, T. Duan, X. Chen, R. Li and T. Peng, ACS Sustainable Chem. Eng., 2014, 2, 718 CrossRef CAS.
- S. Ito, S. M. Zakeeruddin, R. Humphrey-Baker, P. Liska, R. Charvet, P. Comte, M. Takata, H. Miura, S. Uchida and M. Grätzel, Adv. Mater., 2006, 18, 1202 CrossRef CAS; K. Hara, T. Sato, R. Katoh, A. Furube, Y. Ohga, A. Shinpo, S. Suga, K. Sayama, H. Sugihara and H. Arakawa, J. Phys. Chem. B, 2003, 107, 597 CrossRef.
- Z. J. Ning, Y. C. Zhou, Q. Zhang, D. G. Ma, J. J. Zhang and H. Tian, J. Photochem. Photobiol., A, 2007, 192, 8 CrossRef CAS.
- Y.-J. Cheng, S.-H. Yang and C.-S. Hsu, Chem. Rev., 2009, 109, 5868 CrossRef CAS PubMed; N. Kleinhenz, L. Yang, H. Zhou, S. C. Price and W. You, Macromolecules, 2012, 44, 872 CrossRef.
- S. Paek, H. Choi, H. Choi, C.-W. Lee, M.-S. Kang, K. Song, M. K. Nazeeruddin and J. Ko, J. Phys. Chem. C, 2010, 114, 14646 CAS.
- M. Grätzel, Platinum Met. Rev., 1994, 38, 151 Search PubMed.
- M. K. Nazeeruddin, F. D. Angelis, S. Fantacci, A. Selloni, G. Viscardi, P. Liska, S. Ito, B. Takeru and M. Grätzel, J. Am. Chem. Soc., 2005, 127, 16835 CrossRef CAS PubMed.
- B. Wenger, M. Grätzel and J. E. Moser, J. Am. Chem. Soc., 2005, 127, 12150 CrossRef CAS PubMed.
- Y. Hao, X. C. Yang, M. Z. Zhou, J. Y. Cong, X. N. Wang, A. Hagfeldt and L. Sun, ChemSusChem, 2011, 4, 1601 CrossRef CAS PubMed.
- R. Chen, X. Yang, H. Tian, X. Wang, A. Hagfeldt and L. Sun, Chem. Mater., 2007, 19, 4007 CrossRef CAS; K. Sirithip, N. Prachumrak, R. Rattanawan, T. Keawin, T. Sudyoadsuk, S. Namuangruk, S. Jungsuttiwong and V. Promarak, Chem.–Asian J., 2015, 10, 882 CrossRef PubMed.
- Z. S. Wang, Y. Cui, Y. Danoh, C. Kasada, A. Shinpo and K. Hara, J. Phys. Chem. C, 2007, 111, 7224 CAS.
- F. Fabregat-Santiago, J. Bisquert, G. Garcia-Belmonte, G. Boschloo and A. Hagfeldt, Sol. Energy Mater. Sol. Cells, 2005, 87, 117 CrossRef CAS.
- P. Shen, X. Liu, S. Jiang, L. Wang, L. Yi, D. Ye, B. Zhao and S. Tan, Dyes Pigm., 2012, 92, 1042 CrossRef CAS.
- H. Oka, M. Terane, Y. Kiyohara and H. Tanaka, Polyhedron, 2007, 26, 1895 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision A.1, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
- N. Koide and L. Han, Rev. Sci. Instrum., 2004, 75, 2828 CrossRef CAS.
- S. Ito, H. Matsui, K. Okada, S. Kusano, T. Kitamura, Y. Wada and S. Yanagida, Sol. Energy Mater. Sol. Cells, 2004, 82, 421 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: More theoretical calculation data and 1H and 13C NMR spectra. See DOI: 10.1039/c6ra06220b |
| This journal is © The Royal Society of Chemistry 2016 |