Fluorescent carbon dots from milk by microwave cooking†
Abstract
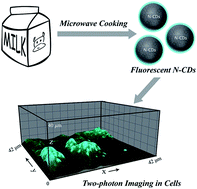
Milk, containing carbohydrates and proteins, is one of the most popular sources of nutrition for human beings. Along with others, we have recently found that milk can also be used as a carbon source for the synthesis of carbon quantum dots. Here, we report the formation of nanometre-sized, highly-fluorescent, nitrogen-doped carbon dots from milk by microwave cooking. Carbon dots thus produced are well dispersed in aqueous solution, and could be easily taken up by HeLa cells without additional surface functionalization. The distribution of carbon dots in cells is confirmed by two-photon excited fluorescence intensity imaging and fluorescence lifetime imaging. Fortunately, no significant cytotoxicity of the milk-derived carbon dots was observed while the strong fluorescence makes them potentially useful for biomedical imaging and many other applications.

 Please wait while we load your content...
Please wait while we load your content...