Poly(norbornyl-NDIs) as a potential cathode-active material in rechargeable charge storage devices†
Abstract
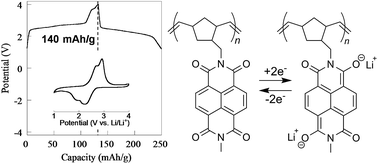
Two pendant-type naphthalene diimide (NDI) polymers bearing a polynorbornene backbone were prepared and their electrochemical properties explored. The NDI-based polymers displayed reversible redox responses in 1 M LiClO4 γ-butyrolactone electrolyte. Polymer 1, bearing a N-methyl group, exhibited remarkable charge-storage capabilities which demonstrated its potential usefulness as an organic cathode-active material in rechargeable devices with excellent performances such as large charge capacity, high rate capability, and charge–discharge cyclability.

 Please wait while we load your content...
Please wait while we load your content...