Mineralization of anionic dyes over visible light responsive Cd(x)Zn(y)S–Nb2O5 heterostructured photocatalysts†
Sachin G. Ghugala,
Suresh S. Umare*a and
Rajamma Sasikala*b
aDepartment of Chemistry, Visvesvaraya National Institute of Technology, Nagpur 440 010, India. E-mail: ssumare@chm.vnit.ac.in; Fax: +91 22 25505151; Tel: +91 22 25590289
bChemistry Division, Bhabha Atomic Research Centre Trombay, Mumbai-400 085, India. E-mail: sasikala@barc.gov.in; Fax: +91 712 2223230; Tel: +91 712 2801316
First published on 28th June 2016
Abstract
A novel, CdxZnyS–Nb2O5 heterostructured photocatalyst is found to have enhanced photocatalytic activity for the degradation and mineralization of a variety of anionic dyes such as acid violet 7, methyl orange and indigo carmine compared to its single phase constituents. This composite catalyst exhibits adsorption enhanced photocatalytic activity and it is found to degrade relatively high concentrations of AV 7 solution (120 ppm) in nearly 45 min using a fluorescent lamp as a source of light (UV < 3%), which has not been reported so far. In this composite system, CdxZnyS exists as a solid solution and both phases, CdxZnyS and Nb2O5 exist in a dispersed state. The composite system exhibits improved visible light absorption compared to pure CdS. Photoluminescence and time resolved fluorescence studies indicate an increased lifetime for the photogenerated charge carriers in the composite compared to CdS and Nb2O5. The improved activity of the composite is attributed to increased lifetime of the charge carriers as well as to the increased adsorption of the dyes on the composite catalysts. A slight, gradual decrease in the photocatalytic activity is observed during repeated cycles of degradation experiment but the catalyst regains its activity after heat treatment. Based on the experiments with different quenchers, it is concluded that the photogenerated holes play a significant role in the degradation reaction.
1. Introduction
Water pollution is one of the serious problems which affect aquatic and terrestrial life. The waste water generated from textile industries contain several dyes, which constitute one of the major groups of organic pollutants.1 Photocatalytic degradation using semiconductors is one of the potential methods to treat these effluents.2,3 This process is attractive as solar radiation can be used for this purpose which is a renewable energy source. As about 45% of solar spectrum contains visible light, development of visible light responsive photocatalysts is a promising area of research.CdS is a II–VI group semiconductor with a direct band gap of 2.4 eV and is widely studied for its photocatalytic properties.4,5 It has excellent visible light absorption property and is suitable for solar energy utilization. However, photocatalytic activity of single phase CdS is poor as recombination of photogenerated charge carriers occurs very fast which limits its practical application. Besides, CdS is unstable and photocorrosion may occur during photocatalytic reaction.6,7 Different strategies adopted to overcome these limitations are making a heterostructure of CdS with other semiconductors,8,9 polymer10 and/or depositing a noble metal co-catalyst.11
Making a heterostructure of CdS with another semiconductor is one of the possible ways which can improve photocatalytic activity and stability of CdS. Further, doping with a suitable metal ion can extent the visible light response to longer wavelength region and improve the photocatalytic activity. In order to improve the stability and photocatalytic activity of CdS, it is coupled with ZnO,12–14 ZrO2,15 SnO2,16,17 Ta2O5,18,19 TiO2,20,21 CdO,22,23 polyaniline,10,24 MoS2 (ref. 25–27) and zeolite.28,29 The resultant composite systems exhibit enhanced photocatalytic activity due to the increased lifetime of the photogenerated charge carriers or due to the increased surface area or both. CdS–Nb2O5 core–shell structure is studied for the photocatalytic degradation of methylene blue under UV-visible irradiation.30 It is reported that Nb2O5 in the composite enhances the photocatalytic activity by decreasing the bandgap and producing strong oxidants like OH radicals. CdS modified with a film of Nb2O5 on the surface show improved photoelectrochemical properties.31 The reason attributed to the increased performance is the stability of CdS photoanode resulting from the surface passivation of CdS by Nb2O5 film. Nb2O5 is an n type semiconductor with band gap energy 3.2 eV.32–35 Advantages of using Nb2O5 are that it has good chemical stability, non toxicity and easy availability.
In the present work, we report the photocatalytic activity of a new combination of a heterostructured Zn doped CdS dispersed on Nb2O5 catalyst for the degradation and mineralization of anionic dyes. It is expected that this combination of the photocatalysts can increase the lifetime of photogenerated charge carriers and enhance the photocatalytic activity. This catalyst is found to degrade higher concentrations of anionic dyes (120 ppm) in about 45 min. This is the first report on adsorption enhanced photocatalytic activity of a heterostructured CdxZnyS–Nb2O5 catalyst for such high concentrations of dye solutions. The observed photocatalytic activity of this heterostructured catalyst is correlated with its various physico-chemical properties.
2. Experimental
2.1. Materials
Niobium(V) oxide (Sigma Aldrich, 99.85%, metal basis), cadmium chloride hydrated AR (S.D. Fine Chem. Ltd. 99.5%), sodium sulfide flakes purified (anhydrous), zinc acetate (Merck, 99.999%, trace metal basis), thiourea GR (Merck, 99%), acid violet 7 (AV 7), indigo carmine (IC) and methyl orange (MO) dye (Sigma-Aldrich) were of analytical grade and used without further purification. Millipore water was used in all solutions.2.2. Synthesis of photocatalysts
Pristine CdS was prepared by treating cadmium chloride with sodium sulfide in ethylene glycol medium.15 Aqueous solution of cadmium chloride (4 g) was mixed with EG (total volume = 50 cm3, water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EG = 1
EG = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume) and refluxed at 100 °C for 15 minutes. At this stage, sodium sulfide (2 g) dissolved in water and EG (50 cm3, water
1 by volume) and refluxed at 100 °C for 15 minutes. At this stage, sodium sulfide (2 g) dissolved in water and EG (50 cm3, water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EG = 1
EG = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume) was added to the solution and refluxed at 120 °C for 4 h. The precipitate obtained was separated, washed and dried in an oven at 90 °C for 5 h followed by calcination in air at 350 °C for 2 h. ZnS was prepared by adopting the same procedure as mentioned above by taking zinc acetate as a Zn source. For the synthesis of CdS–Nb2O5 composite analytical grade Nb2O5 was used. Different amounts of CdS (40, 60, 70, 80, 90 and 96% by weight of Nb2O5, named as xxCdS–Nb where xx represents the percentage of CdS) on Nb2O5 were loaded by an impregnation method. Aqueous cadmium chloride solution was stirred with Nb2O5 powder and evaporated to dryness (80–90 °C). The cadmium chloride adsorbed on Nb2O5 was heated with an aqueous solution of thiourea (Cd
1 by volume) was added to the solution and refluxed at 120 °C for 4 h. The precipitate obtained was separated, washed and dried in an oven at 90 °C for 5 h followed by calcination in air at 350 °C for 2 h. ZnS was prepared by adopting the same procedure as mentioned above by taking zinc acetate as a Zn source. For the synthesis of CdS–Nb2O5 composite analytical grade Nb2O5 was used. Different amounts of CdS (40, 60, 70, 80, 90 and 96% by weight of Nb2O5, named as xxCdS–Nb where xx represents the percentage of CdS) on Nb2O5 were loaded by an impregnation method. Aqueous cadmium chloride solution was stirred with Nb2O5 powder and evaporated to dryness (80–90 °C). The cadmium chloride adsorbed on Nb2O5 was heated with an aqueous solution of thiourea (Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thiourea = 1
thiourea = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio, at 80–90 °C). The resultant mixture was evaporated to dryness followed by heating the powder at 350 °C for 2 h. Zn doped CdS–Nb2O5 (% by weight of CdS) composites was prepared by taking calculated amounts of zinc acetate and cadmium chloride in water and adopting the same procedure as that for CdS–Nb2O5 composites. The composition of 80CdS–Nb was chosen for Zn doping. Different compositions synthesized are 20, 40, 60, 80 and 96% by weight of CdS in the composite which are named as 80CdxxZnS–Nb where xx represents the amount of Zn taken.
4 ratio, at 80–90 °C). The resultant mixture was evaporated to dryness followed by heating the powder at 350 °C for 2 h. Zn doped CdS–Nb2O5 (% by weight of CdS) composites was prepared by taking calculated amounts of zinc acetate and cadmium chloride in water and adopting the same procedure as that for CdS–Nb2O5 composites. The composition of 80CdS–Nb was chosen for Zn doping. Different compositions synthesized are 20, 40, 60, 80 and 96% by weight of CdS in the composite which are named as 80CdxxZnS–Nb where xx represents the amount of Zn taken.
2.3. Photocatalytic experiments
The photocatalytic activity of the prepared catalysts was evaluated by studying the degradation of AV 7 (50 or 120 ppm), methyl orange (MO) and indigo carmine (IC) in aqueous solution under sunlight type irradiation. Photodegradation studies of AV 7 dye were carried out in a homemade photoirradiator consisting of day light compact fluorescent lamps (12 lamps, 100 W, Oreva) as source of radiation. The radiation from these lamps contains mainly visible light along with a very small amount of UV light (<3%).36,37 The irradiation chamber consists of a rectangular galvanised iron box containing fluorescent lamps arranged vertically and equidistantly in the chamber with aluminium foil on the walls of box as a reflecting media. Air circulation was carried out to minimize the heating effect. The average light flux measured by Lutron lux meter (model LX-101) was found out to be 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–65
000–65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 lux. For each experiment, 125 ml of dye (50 or 120 ppm) solution was taken in a beaker containing 0.35 g of catalyst suspended in it. Prior to irradiation, the catalyst was immersed in the dye solution for 30 min to attain the adsorption–desorption equilibrium at ambient conditions. During the experiment, magnetic stirring was performed for the homogenization of suspension. The photocatalytic activity was measured by taking a small volume of clear supernatant liquid at regular time intervals, centrifuged it and analyzed with UV-visible spectrophotometer (Shimadzu, UV-1800). The concentration of the solution was monitored by taking its absorbance at λmax = 523 nm. The TOC values of degraded dye samples at different time intervals were monitored with the help of TOC analyser (Shimadzu, TOC-L).
000 lux. For each experiment, 125 ml of dye (50 or 120 ppm) solution was taken in a beaker containing 0.35 g of catalyst suspended in it. Prior to irradiation, the catalyst was immersed in the dye solution for 30 min to attain the adsorption–desorption equilibrium at ambient conditions. During the experiment, magnetic stirring was performed for the homogenization of suspension. The photocatalytic activity was measured by taking a small volume of clear supernatant liquid at regular time intervals, centrifuged it and analyzed with UV-visible spectrophotometer (Shimadzu, UV-1800). The concentration of the solution was monitored by taking its absorbance at λmax = 523 nm. The TOC values of degraded dye samples at different time intervals were monitored with the help of TOC analyser (Shimadzu, TOC-L).
2.4. Characterization
X-ray powder diffraction (XRD) patterns were recorded using a X'Pert PRO PANalytical X-ray diffractometer (Philips, Holland) operating at 40 kV. Cu Kα radiation (α = 1.5418 Å) was used for this purpose. The surface area was determined by Brunauer–Emmet–Teller (BET) method using Micromeritics ASAP 2020 V3.04 H surface area analyzer by nitrogen adsorption at 77 K. The TEM images were taken using a FEI Tecnai T-20 electron microscope operating at 300 kV. SEM and energy dispersive X-ray spectroscopy (EDS) analysis was carried out using FEI Quanta 200 ESEM. UV-visible diffuse reflectance spectra (UV-vis DRS) of all samples were recorded using a Jasco (model V-670) spectrophotometer equipped with an integrating sphere accessory. Barium sulfate was used as reference for the reflectance spectra. Photoluminescence (PL) spectra were recorded using FLSP 920 (Edinburgh Instruments) equipped with a 450 W Xe arc lamp as the excitation source and a red sensitive Peltier element cooled Hamamatsu R2658 PMT as the detector. Excited state lifetimes of the charge carriers were measured with the same instrument using a nanosecond hydrogen flash lamp as the excitation source and employing Time Correlated Single Photon Counting (TCSPC) technique. A reconvolution procedure was used to find out the fluorescence lifetime.3. Results and discussion
3.1. Phases and morphological studies
Fig. 1A shows the XRD patterns for CdS, ZnS, Nb2O5, 80CdS–Nb and 80Cd80ZnS–Nb catalysts. A small portion of the XRD patterns in the region of 20 to 35 degree is shown in Fig. 1B so that the peak positions of some of the intense peaks of different phases can be seen clearly. XRD pattern of CdS suggests that it is monophasic in nature and have wurtzite type (hexagonal) structure with no other impurity phase (JCPDS file no. 41-1049). Niobium oxide exists as biphasic containing orthorhombic (JCPDS file no. 71-0336) and monoclinic phases (JCPDS file no. 37-1468). XRD pattern of 80CdS–Nb shows peaks corresponding to both CdS and Nb2O5, suggesting the presence of both phases in the composite sample. The pattern of 80Cd80ZnS–Nb shows peaks of CdS and Nb2O5 phases and no peaks of ZnS is seen. It may be noticed from Fig. 1B that the peaks corresponding to CdS for 80Cd80ZnS–Nb sample are shifted to higher angles indicating that a solid solution of cadmium zinc sulfide is formed. XRD pattern of ZnS suggest that both hexagonal (JCPDS file no. 75-1547) and cubic phase (77-2100) co-exist in ZnS. The BET surface areas of CdS, ZnS, Nb2O5, 80CdS–Nb and 80Cd80ZnS–Nb catalysts are given in Table 1. It is seen that the surface areas of ZnS and 80Cd80ZnS–Nb are comparable and higher than that of CdS, Nb2O5 and 80CdS–Nb. | ||
| Fig. 1 (A) XRD patterns for CdS, ZnS, Nb2O5, 80CdS–Nb and 80Cd80ZnS–Nb catalysts. (B) Enlarge view of XRD pattern in the range of 2θ (20–35°). | ||
| S. N. | Catalysts | Rate k (min−1), 30 min | Degradation efficiency (%), 30 min | Band gap (eV) | Surface area (m2 g−1) |
|---|---|---|---|---|---|
| 1 | CdS | 0.004 | 18 | 2.38 | 1 |
| 2 | Nb2O5 | — | — | 3.12 | 3 |
| 3 | ZnS | — | 11 | 3.37 | 12 |
| 4 | 80CdS–Nb | 0.011 | 39 | 2.34 | 2 |
| 5 | 80Cd80ZnS–Nb | 0.057 | 98 | 2.31 | 15 |
SEM images of CdS, Nb2O5, 80CdS–Nb and 80Cd80ZnS–Nb are shown in Fig. S1 of ESI.† It can be seen that the particles of all samples are irregular shaped and the particles appears to be aggregated nanocrystallites. To see the distribution of different elements in Cd80Zn80S–Nb heterostructures, energy dispersive X-ray spectroscopy (EDS) with elemental mapping was performed. The mapping results of selected area of SEM image (Fig. S2†) suggest the coexistence of Zn, Cd, S, O and Nb elements in the heterostructure. The mapping clearly shows that the distribution of all the elements is almost homogeneous and uniform. TEM image, SAED pattern and HRTEM of 80CdS–Nb and 80Cd80ZnS–Nb are shown in Fig. 2A and B respectively. It can be seen from the image of 80CdS–Nb that the particle size of the composite is in the range of 70–150 nm. HRTEM and SAED pattern indicate the presence of both Nb2O5 (orthorhombic) and CdS (hexagonal) phases. HRTEM suggests that the particles of both phases are well mixed in the composite. The SAED pattern could be indexed as hexagonal wurtzite phase of CdS and orthorhombic phase of Nb2O5. The particle size of 80Cd80ZnS–Nb as seen from Fig. 2B is ∼100–150 nm. The distance between the fringes of HRTEM corresponds to CdS (hexagonal) and Nb2O5 (orthorhombic) and they appear to be in a dispersed state. The SAED pattern showed the presence of both CdS and Nb2O5. The dot patterns of the SAED of both samples suggest that they are single crystalline in nature. TEM image and SAED pattern of CdS is shown in Fig. S3 of ESI.† It can be concluded from the figure that the particle size of CdS is in the range of 70–100 nm. SAED pattern is indexed as the hexagonal wurtzite phase of CdS.
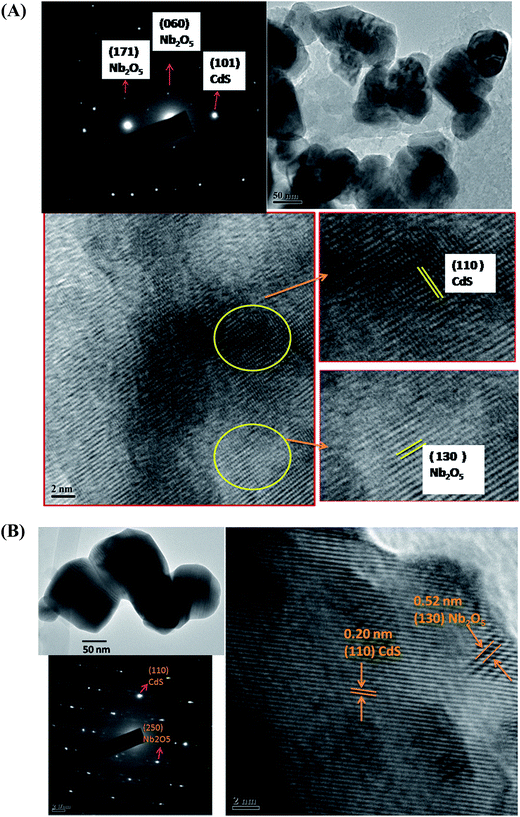 | ||
| Fig. 2 (A) TEM image, SAED pattern and HRTEM of 80CdS–Nb. (B) TEM image, SAED pattern and HRTEM of 80Cd80ZnS–Nb. | ||
3.2. Optical properties
Fig. 3A shows the UV-visible diffuse reflectance spectra of CdS, Nb2O5, ZnS, 80CdS–Nb and 80Cd80ZnS–Nb. The absorption edges of the composite samples are in between that of CdS and Nb2O5. 80Cd80ZnS–Nb composite shows the onset of absorption in higher wavelength region compared to CdS and 80CdS–Nb. Fig. 3B shows the plot of (F(R)hν)2 vs. photon energy. The bandgap energies obtained from this plot for CdS, ZnS, Nb2O5, 80CdS–Nb, Cd80Zn80S–Nb are 2.38, 3.37, 3.12, 2.34 and 2.31 respectively. The slightly decreased bandgap of the composite compared to pristine CdS can be due to the increased number of defect levels/surface states present in these samples. The decreased bandgap of the composite indicates that the heterostructured photocatalysts can utilize more visible light for the photocatalytic reaction.38 | ||
| Fig. 3 (A) UV-visible diffuse reflectance spectra of CdS, Nb2O5, ZnS, 80CdS–Nb and 80Cd80ZnS–Nb. (B) Kubelka–Munk plot for band gap calculation of CdS, Nb2O5, ZnS, 80CdS–Nb and 80Cd80ZnS–Nb. | ||
PL spectra of CdS, Nb2O5, 80CdS–Nb and 80Cd80ZnS–Nb are shown in Fig. 4A. The spectrum of CdS shows a broad peak in the region of 500–650 nm and centred around 570 nm. As the bandgap of this material is 2.38 eV, this cannot be due to band edge emission. This peak is originating from the defect levels/surface states and can be assigned to the defect emission.39 Nb2O5 show an emission peak having a peak maximum at 430 nm and this peak also corresponds to defect emission. Spectra of the composites exhibit features of both Nb2O5 and CdS confirming that CdS and Nb2O5 exist as separate phases. 80CdS–Nb and 80Cd80ZnS–Nb show two peaks around 430 and 570 nm corresponding to CdS and Nb2O5 respectively. Fig. 4B shows the fluorescence emission decay curves corresponding to CdS, 80Cd80ZnS–Nb samples obtained after excitation at 282 nm and monitoring the emission at emission maximum at 570 nm. Fluorescence emission at 570 nm was studied as it is originating from CdS, which is the efficient photoactive material in this system. The lifetime values are τ = 1.2 and 5.8 ns for CdS and 80Cd80ZnS–Nb respectively. Higher lifetime of 80Cd80ZnS–Nb sample confirms better separation of charge carriers and lesser extent of non-radiative quenching in the composite compared to single phase CdS.
 | ||
| Fig. 4 (A) PL spectra of CdS, Nb2O5, 80CdS–Nb and 80Cd80ZnS–Nb. (B) Fluorescence emission decay curves of CdS and 80Cd80ZnS–Nb. | ||
3.3. Photocatalytic activity
Fig. S4† shows the effect of CdS loading on Nb2O5 for photocatalytic degradation of AV 7 (concentration: 50 ppm). It can be seen that 80 and 90% concentrations show almost similar activity. The photocatalytic activity decreases when the concentration is increased from 90% to 96%. Among these, 80% CdS was chosen for further study as this is found to be the optimum concentration of CdS on Nb2O5 to get higher efficiency. Zn doping was performed in order to further improve the photocatalytic activity of the optimised 80CdS–Nb composite. Fig. S5† shows the effect of Zn doping in optimised 80CdS–Nb composite. It is clear from the figure that as the Zn loading increases the adsorption of AV 7 (50 ppm) increases. Among the prepared composites, 80Cd80ZnS–Nb shows better photocatalytic activity than the other compositions. As 80Cd80ZnS–Nb shows very high adsorption of the dye, a higher concentration of AV 7 (120 ppm) was taken to evaluate the photocatalytic performance. Fig. 5A shows the photocatalytic activity of CdS, Nb2O5, 80CdS–Nb and 80Cd80ZnS–Nb for the degradation of AV 7 (concentration: 120 ppm) dye. It can be seen that a significant amount of the dye is adsorbed by the catalyst under the dark conditions and when it is irradiated, the adsorbed dye undergoes degradation on the surface of the catalyst. 80Cd80ZnS–Nb shows the best performance for photodegradation of AV 7 (120 ppm). In order to confirm whether the reaction is mineralization of the dye and it is not just the decolourisation, the total organic carbon (TOC) of the solution was analyzed as a function of time. The results are shown in Fig. 5B for the 80Cd80ZnS–Nb catalyst for the photocatalytic reaction of 120 ppm of AV 7 dye. It can be seen that the TOC of the solution decreases as the reaction time increases and reaches almost to zero after 45 min suggesting that mineralization of the dye occurs during the photocatalytic reaction. Among the different composites, 80Cd80ZnS–Nb shows enhanced reaction rate and degradation efficiency than that of CdS, ZnS, Nb2O5 and 80CdS–Nb. The degradation efficiency and the rate constant calculated for the initial 30 min for different catalysts are presented in Table 1. The results indicate higher reaction rate over 80Cd80ZnS–Nb catalyst and a degradation efficiency of 98% is obtained within 30 minutes of irradiation for a dye solution of concentration of 120 ppm. | ||
| Fig. 5 (A) Photocatalytic performance of prepared catalysts for 120 ppm AV 7. (B) Total organic carbon (TOC%) during photocatalytic degradation reaction of AV 7 by 80Cd80ZnS–Nb. | ||
The reaction kinetics was studied by applying the Langmuir–Hinshelwood model. Fig. S6† shows the kinetics of AV 7 degradation in presence of Cd80Zn80S–Nb. It was observed that the obtained experimental data follows the Langmuir–Hinshelwood kinetics which follows the pseudo first order rate equations. A linear relationship between ln(C0/C) vs. irradiation time (min) was observed for AV 7 (concentration: 120 ppm) degradation over 80Cd80ZnS–Nb. The pseudo-first-order rate constant and linear regression coefficient (R2 = 0.998) for photodegradation of AV 7 with Cd80Zn80S–Nb was obtained from Fig. S6.† The observed photodegradation rate (k, 45 min) by 80Cd80ZnS–Nb was found out to be 0.054 min−1.
The dye AV 7 has a relatively strong adsorption on the 80Cd80ZnS–Nb catalyst at room temperature. For the purpose of the present study, the experiments were performed with different concentration of AV 7 dye solution at room temperature. Fig. S7(A)† shows the adsorption isotherm of AV 7 from aqueous solution at room temperature on the 80Cd80ZnS–Nb catalysts where the amount of equilibrium adsorption, qe is plotted as a function of equilibrium concentration in the bulk solution, Ce. It is clear from Fig. S7† that the curve demonstrates the Langmuir type behaviour.40–42 The adsorption parameters, qm (equilibrium adsorption capacity) and KL (Langmuir adsorption constant) was obtained from the slope and intercept of the plot Ce/qe versus Ce (the linear regression coefficients for curves is 0.998). The qm and KL value for linear fit is found out to be 20 mg g−1 and 0.0453 mg l−1 respectively. The result suggests that in the concentration range studied the Langmuir model can be used to describe the adsorption process of AV 7 on 80Cd80ZnS–Nb catalysts.
The increased activity of 80CdS–Nb can be attributed to the increased lifetime of the charge carriers in the composite compared to single phase CdS. As the conduction band (CB) potential of Nb2O5 is less negative than that of CdS, the photogenerated electrons from CdS can be transferred to the CB of Nb2O5. This electron transfer minimizes the charge recombination and increases the lifetime of the photogenerated charge carriers. This charge transfer is very effective when these two phases are in a highly dispersed state, which brings about good physical contact with each other. The EDS elemental mapping indicates that these two phases are in a dispersed state (Fig. S2†), which can favour the charge transfer process. The observed improvement of photocatalytic activity with Zn doping can be attributed to three reasons. First reason is that the adsorption of the dye on the catalyst is enhanced as a result of Zn doping. Charge transfer is facilitated if the dye is adsorbed on the catalyst and hence the photocatalytic activity increases. Secondly, the increased lifetime of the charge carriers in the composite increases the photocatalytic activity. A similar observation of high adsorption of dye on the surface of Zn1−xCdxS has been reported earlier.40,41,43 The third reason is the improved visible light absorption exhibited by the Zn doped composite.
The photocatalytic activity of Cd80Zn80S–Nb for AV 7 (120 ppm) was also studied at different conditions like varying pH and catalysts loading. Fig. S8† shows the photocatalytic activity at different pH and the results suggest that the 80Cd80ZnS–Nb catalysts can work efficiently with varied pH conditions and mineralize the dye (Fig. S9†). Fig. S10† shows the effect of Cd80Zn80S–Nb loading on photocatalytic activity. The results suggest that 0.35 and 0.45 g of catalyst can be used for efficient degradation reaction and increasing the concentration above this leads to decrease in the efficiency. TOC obtained for these experiments as a function of time is shown in Fig. S11,† which suggests that the dye was effectively mineralised with 0.35 g catalysts loading. In order to further confirm that it is not just adsorption, but photocatalytic degradation is also taking place, the experiment was conducted using higher concentration of AV 7 dye. The results (Fig. S12 and S13†) suggest that the dye was effectively degraded and mineralized after 120 min of irradiation indicating that the dye is getting mineralized through photocatalytic reaction and not just getting adsorbed on the surface.
3.4. Photocatalytic degradation of other anionic dyes
The photocatalytic activity of 80Cd80ZnS–Nb for the degradation and mineralization of other anionic dyes were also tested. For this experiment, a mixture of dyes (∼42 ml) each of AV 7, MO and IC (concentration: 50 ppm) was used and irradiated under the same conditions mentioned earlier. The UV-visible absorption spectra of the dye solution before and after irradiation for different time periods are shown in Fig. 8A. The spectra indicate that almost complete degradation of all three dyes takes place after irradiation for 120 minutes. This result suggests that the catalyst, 80Cd80ZnS–Nb, is efficient for the degradation of anionic dyes. TOC present in the solution was also tested as a function of time. It is seen from Fig. 8B that 95% decrease of TOC occurs after 150 min of irradiation.4. Conclusions
A composite of Zn doped CdS and Nb2O5 is found to be an efficient catalyst for the photodegradation and mineralization of relatively higher concentrations of anionic dyes like AV 7, MO and IC. The photocatalytic activity of the Zn doped CdS–Nb2O5 composite is found to be higher than that of single phase CdS, Nb2O5 and CdS–Nb2O5 composite. In the composite, both CdS and Nb2O5 exist in a highly dispersed state. Improved visible light absorption is exhibited by CdS–Nb2O5 as a result of Zn doping, which can be due to the increased number of defect levels/surface states present in these samples. It is concluded from the PL spectra that a number of surface states/defect levels are present in the composite. Increased lifetime for the photogenerated charge carriers is observed for the composite as compared to single phase CdS. The enhanced photocatalytic activity of the Zn doped CdS–Nb2O5 composite is attributed to factors like, increased lifetime of the photogenerated charge carriers, increased adsorption of dyes on the catalyst and the improved visible light absorption. A slight decrease in the photocatalytic activity is observed during repeated cycles of degradation experiment, but the catalyst regained its activity fully after regeneration. Though, all three species like photogenerated holes (h+), super oxide radicals anions (O2˙−) and hydroxyl radicals (OH˙) play role in the degradation reaction, the photogenerated holes (h+) influence the reaction more strongly.Acknowledgements
One of the authors, Sachin G. Ghugal is thankful to Visvesvaraya National Institute of Technology (VNIT), Nagpur for awarding the Ph.D fellowship. Suresh S. Umare is thankful to DST-SERB for financial assistance through project number SB/EMEQ-052/2014 SERB.References
- H. Langhals, Angew. Chem., Int. Ed., 2004, 43, 5291–5292 CrossRef CAS.
- Z. Wang, W. Ma, C. Chen, H. Ji and J. Zhao, Chem. Eng. J., 2011, 170, 353–362 CrossRef CAS.
- R. Yuan, S. N. Ramjaun, Z. Wang and J. Liu, Chem. Eng. J., 2012, 192, 171–178 CrossRef CAS.
- H. Zhu, R. Jiang, L. Xiao, Y. Chang, Y. Guan, X. Li and G. Zeng, J. Hazard. Mater., 2009, 169, 933–940 CrossRef CAS PubMed.
- N. Bao, L. Shen, T. Takata and K. Domen, Chem. Mater., 2008, 20, 110–117 CrossRef CAS.
- G. C. De, A. M. Roy and S. S. Bhattacharya, Int. J. Hydrogen Energy, 1995, 20, 127–131 CrossRef CAS.
- W. Li, D. Li, Z. Chen, H. Huang, M. Sun, Y. He and X. Fu, J. Phys. Chem. C, 2008, 112, 14943–14947 CAS.
- V. M. Daskalaki, M. Antoniadou, G. Li Puma, D. I. Kondarides and P. Lianos, Environ. Sci. Technol., 2010, 44, 7200–7205 CrossRef CAS PubMed.
- B. Huang, Y. Yang, X. Chen and D. Ye, Catal. Commun., 2010, 11, 844–847 CrossRef CAS.
- H. Zhang and Y. Zhu, J. Phys. Chem. C, 2010, 114, 5822–5826 CAS.
- L.-L. Ma, H.-Z. Sun, Y.-G. Zhang, Y.-L. Lin, J.-L. Li, E.-k. Wang, Y. Yu, M. Tan and J.-B. Wang, Nanotechnology, 2008, 19, 115709 CrossRef PubMed.
- H. Zhao, Y. Dong, P. Jiang, G. Wang, H. Miao, R. Wu, L. Kong, J. Zhang and C. Zhang, ACS Sustainable Chem. Eng., 2015, 3, 969–977 CrossRef CAS.
- S. Khanchandani, S. Kundu, A. Patra and A. K. Ganguli, J. Phys. Chem. C, 2012, 116, 23653–23662 CAS.
- M. Zhou, Y. Hu, Y. Liu, W. Yang and H. Qian, CrystEngComm, 2012, 14, 7686–7693 RSC.
- R. Sasikala, A. R. Shirole, V. Sudarsan, K. G. Girija, R. Rao, C. Sudakar and S. R. Bharadwaj, J. Mater. Chem., 2011, 21, 16566–16573 RSC.
- S. G. Ghugal, S. S. Umare and R. Sasikala, Appl. Catal., A, 2015, 496, 25–31 CrossRef CAS.
- Y. Liu, P. Zhang, B. Tian and J. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 13849–13858 CAS.
- S. G. Ghugal, S. S. Umare and R. Sasikala, Mater. Res. Bull., 2015, 61, 298–305 CrossRef CAS.
- L. Xu, W. Shi and J. Guan, Catal. Commun., 2012, 25, 54–58 CrossRef CAS.
- W. Dong, F. Pan, L. Xu, M. Zheng, C. H. Sow, K. Wu, G. Q. Xu and W. Chen, Appl. Surf. Sci., 2015, 349, 279–286 CrossRef CAS.
- Z. Chen and Y.-J. Xu, ACS Appl. Mater. Interfaces, 2013, 5, 13353–13363 CAS.
- W. Yang, Y. Liu, Y. Hu, M. Zhou and H. Qian, J. Mater. Chem., 2012, 22, 13895–13898 RSC.
- S. V. Kahane, R. Sasikala, B. Vishwanadh, V. Sudarsan and S. Mahamuni, Int. J. Hydrogen Energy, 2013, 38, 15012–15018 CrossRef CAS.
- K. He, M. Li and L. Guo, Int. J. Hydrogen Energy, 2012, 37, 755–759 CrossRef CAS.
- C. Wang, H. Lin, Z. Xu, H. Cheng and C. Zhang, RSC Adv., 2015, 5, 15621–15626 RSC.
- Y. Min, G. He, Q. Xu and Y. Chen, J. Mater. Chem. A, 2014, 2, 2578–2584 CAS.
- R. Sasikala, A. Gaikwad, O. Jayakumar, K. Girija, R. Rao, A. Tyagi and S. Bharadwaj, Colloids Surf., A, 2015, 481, 485–492 CrossRef CAS.
- R. Sasikala, A. Gaikwad, V. Sudarsan, R. Rao, B. Viswanadh and S. Bharadwaj, Phys. Chem. Chem. Phys., 2015, 17, 6896–6904 RSC.
- A. Nezamzadeh-Ejhieh and Z. Banan, Desalination, 2011, 279, 146–151 CrossRef CAS.
- L. C. Oliveira, H. S. Oliveira, G. Mayrink, H. S. Mansur, A. A. Mansur and R. L. Moreira, Appl. Catal., B, 2014, 152, 403–412 CrossRef.
- A. Pareek, P. Paik and P. H. Borse, Langmuir, 2014, 30, 15540–15549 CrossRef CAS PubMed.
- Y. Zhao, C. Eley, J. Hu, J. S. Foord, L. Ye, H. He and S. C. E. Tsang, Angew. Chem., Int. Ed., 2012, 51, 3846–3849 CrossRef CAS PubMed.
- T. Ohuchi, T. Miyatake, Y. Hitomi and T. Tanaka, Catal. Today, 2007, 120, 233–239 CrossRef CAS.
- A. G. Prado, L. B. Bolzon, C. P. Pedroso, A. O. Moura and L. L. Costa, Appl. Catal., B, 2008, 82, 219–224 CrossRef CAS.
- H.-Y. Lin, H.-C. Yang and W.-L. Wang, Catal. Today, 2011, 174, 106–113 CrossRef CAS.
- V. N. Kuznetsov and N. Serpone, J. Phys. Chem. B, 2006, 110, 25203–25209 CrossRef CAS PubMed.
- C. Belver, R. Bellod, S. Stewart, F. Requejo and M. Fernandez-Garcia, Appl. Catal., B, 2006, 65, 309–314 CrossRef CAS.
- C. Shifu, C. Lei, G. Shen and C. Gengyu, Powder Technol., 2005, 160, 198–202 CrossRef.
- B. Yang, J. E. Schneeloch, Z. Pan, M. Furis and M. Achermann, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 073401 CrossRef.
- Y. Xu and C. H. Langford, Langmuir, 2001, 17, 897–902 CrossRef CAS.
- K. Lv, J. Yu, K. Deng, J. Sun, Y. Zhao, D. Du and M. Li, J. Hazard. Mater., 2010, 173, 539–543 CrossRef CAS PubMed.
- R. Dutta, T. V. Nagarjuna, S. A. Mandavgane and J. D. Ekhe, Ind. Eng. Chem. Res., 2014, 53, 18558–18567 CrossRef CAS.
- W. Wang, W. Zhu and H. Xu, J. Phys. Chem. C, 2008, 112, 16754–16758 CAS.
- N. Zhang, Y. Zhang, M.-Q. Yang, Z.-R. Tang and Y.-J. Xu, J. Catal., 2013, 299, 210–221 CrossRef CAS.
- Y. Zhang, N. Zhang, Z.-R. Tang and Y.-J. Xu, Chem. Sci., 2012, 3, 2812–2822 RSC.
- O. Carp, C. L. Huisman and A. Reller, Prog. Solid State Chem., 2004, 32, 33–177 CrossRef CAS.
- H. Park and W. Choi, J. Phys. Chem. B, 2004, 108, 4086–4093 CrossRef CAS.
- T. M. El-Morsi, W. R. Budakowski, A. S. Abd-El-Aziz and K. J. Friesen, Environ. Sci. Technol., 2000, 34, 1018–1022 CrossRef CAS.
- P. Calza and E. Pelizzetti, Pure Appl. Chem., 2001, 73, 1839–1848 CrossRef CAS.
- Y. Chen, S. Yang, K. Wang and L. Lou, J. Photochem. Photobiol., A, 2005, 172, 47–54 CrossRef CAS.
- P. Raja, A. Bozzi, H. Mansilla and J. Kiwi, J. Photochem. Photobiol., A, 2005, 169, 271–278 CrossRef CAS.
- M. Stylidi, D. I. Kondarides and X. E. Verykios, Appl. Catal., B, 2004, 47, 189–201 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra06023d |
| This journal is © The Royal Society of Chemistry 2016 |



