High quantum yield and pH sensitive fluorescence dyes based on coumarin derivatives: fluorescence characteristics and theoretical study†
Chao-jun Hua,
Kan Zhang,
Ming Xin,
Ting Ying,
Jian-rong Gao,
Jian-hong Jia and
Yu-jin Li*
College of Chemical Engineering, Zhejiang University of Technology, Hangzhou 310014, People's Republic of China. E-mail: lyjzjut@zjut.edu.cn
First published on 3rd May 2016
Abstract
The fluorescence coumarin derivatives were synthesized by an efficient one-pot, three-component reaction in 80–90% yields. These fluorescence dyes exhibited high fluorescent intensity and quantum yield where compound 4e, with p-methyl substituted on the phenyl ring, had the maximum fluorescence intensity and also the fluorescence quantum yield reached 0.83. These coumarin derivatives display different fluorescence in acidity and alkaline conditions and the fluorescent colour changed from blue to yellow-green when the pH of the solution turned from acidic to alkaline. The pH sensitive fluorescence properties of the coumarin derivatives showed a large shift from 441 to 538 nm in wavelength. Experimental results were confirmed with DFT and TDDFT calculations.
Introduction
Coumarins consist of pyrone and benzene1 which are important components of versatile applications in various fields of science and technology2 such as perfumes,3 cosmetics,4 disperse dyes,5 cationic dyes,6 charge-transfer agents,7 solar energy collectors,8 and potential applications in organic light-emitting diodes (OLEDs)9 etc. In addition, coumarin derivatives have played an important role in medicinal chemistry such as the platelet inhibition agent, anti-thrombus agent,10 hepatoprotective drugs, and so on.Coumarins also are well-known laser dyes for the blue-green spectral region11 whose derivatives are one of the most favorable fluorophore groups used to develop fluorescence agents12 because of their high quantum yields and high photostability.13 It was reported14 that the electron donating groups in the 7-position and electron-withdrawing groups in the 3-position were able to enhance the fluorescence intensity of coumarins15 based on an intramolecular charger transfer process.16 Among different coumarin dyes, Xu's group reported that 7-(N,N-diethylamino)coumarin-3-aldehyde was selected as a fluorophore and ionophore for the construction of Cu2+ selective fluorescence sensors.17 In 2006 Li's group reported that 7-aminocoumarin derivative dyes underwent change in their dipole moment on excitation from ground to the excited S1 state transition.18 Thus, these molecules are suitable sensors for investigating many physical and physiochemical processes in a condensed phase,19 making use of their fluorescence properties.
Optical methods20 via fluorescent imaging and sensing are attracting attention for pH measurements due to their rapid response time,21 simplicity, non-invasiveness, and good sensitivity.22 Currently, a large number of fluorescence sensors for pH measurement have been reported in the literature and are commercially available due to their extensive applications. As an example, Liu23 published on coumarin sensors that have optical responses under acid conditions. Their studies disclosed that these coumarin derivatives could be used for quantitative fluorescence imaging in detection of intracellular pH by the formation of an intramolecular charge transfer (ICT) platform in an acidic environment, leading to colour changes. In addition, Oliveira's group24 designed a new ratiometric fluorescent pH coumarin quinoline derivative sensor where in acidic pH it exhibited a strong fluorescence which was very useful for monitoring pH variations from neutral to acidic conditions in living cells. Also, a series of highly fluorescence quantum yield coumarin derivatives were synthesized using a very simple, facile, and economically cheap route which was based on literature from Ahmed's group25 and the optical properties were investigated under various conditions. There was high fluorescent intensity and quantum yield in DMSO. Moreover, we describe a different phenomenon between acid and alkaline conditions in DMSO and we used DFT and TDDFT calculations to confirm the experimental results.
Experimental section
General methods
All chemicals used in the current study were purchased from commercial vendors and used as received without further purification, unless otherwise noted. All solvents were purified and dried using standard methods prior to use. Nuclear magnetic resonance (1H, 13C) spectra were recorded on a Bruker AM 500 spectrometer (Switzerland) with chemical shifts reported as ppm at 500 and 125 MHz, respectively (in DMSO with TMS as the internal standard). Fluorescence spectra were obtained with an F-7000 fluorescence spectrophotometer (Hitachi) in a solution of 1.0 × 10−5 mol L−1. UV-Vis absorption spectra were measured on a UV-2550 (Shimadzu). The absorption and emission studies at different pH were all measured in a DMSO–water mixture (DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 9
H2O = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) by a PHS-3C digital pH-meter (Youke, Shanghai).
1) by a PHS-3C digital pH-meter (Youke, Shanghai).
General procedure for the synthesis of 4-aminocoumarin
A mixture of powdered 4-hydroxycoumarin (1.07 g, 0.0066 mol) and ammonium acetate (7.87 g, 0.100 mol) was melted in an oil bath (max. 130 °C) and stirred for 3 h. After cooling to ambient temperature, water was added and the crude product was isolated as yellow crystals by simple filtration. 4-Aminocoumarin was obtained by recrystallized from EtOH and water as yellow crystals (0.98 g, 92.2%).25General procedure for the synthesis of compound 4a–4e
A mixture of benzaldehyde (0.106 g, 1 mmol), 1,3-cyclohexanedione (0.112 g, 1 mmol) and 4-aminocoumarin (0.161 g, 1 mmol) in acetic acid (6 mL) was stirred for 2–3 h at 110 °C; the completed reaction was detected by TLC analysis and then cooled to room temperature. Then, the reaction mixture was washed with H2O (20 mL) and the crude product was recrystallized from DMSO to give product 4a (0.295 g, 86.3%) as light yellow solid.25Computational methodology
All the structure optimizations were based on density functional theory (DFT) with the B3LYP functional and 6-311G basis set. The fluorescence emission wavelength and the excitation wavelength of the compounds were calculated with the time-dependent density functional theory (TDDFT). All these calculations were performed with Gaussian 09W.26Results and discussion
As shown in Scheme 1, the coumarin fused dihydropyridine derivatives 4 were synthesized by an efficient three-component reaction of 1,3-cyclohexadione and aryl aldehydes and products 4 were obtained in 86% (4a), 90% (4b), 88% (4c), 82% (4d), and 83% (4e) yields, respectively. With a para-nitro substituent on the benzene unit the largest yield (90%) of the desired product was obtained. These compounds were all characterized by 1H-NMR and 13C-NMR.UV-Vis absorption and fluorescence emission spectra
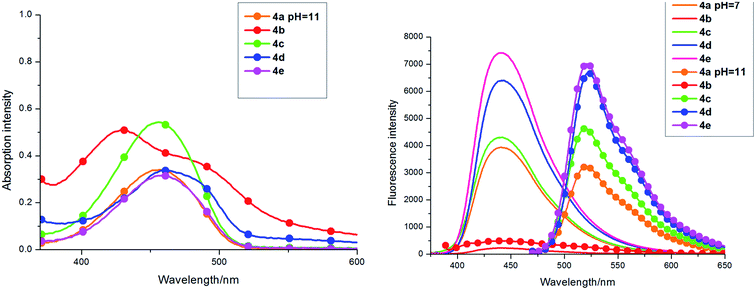
The absorption and fluorescence emission spectra of coumarin-fused dihydropyridine derivatives 4 were detected and summarized in Fig. 1. The fluorescence properties of the coumarin derivatives 4 were examined under different conditions including different organic solvents and with different pH conditions.The solvent effect on absorption and fluorescence properties of 7-(4-methoxyphenyl)-9,10,11,12-tetrahydro-6H-chromeno[4,3-b]quinoline-6,8(7H)-dione (4c) were examined (Fig. 1, Table 1). As shown in Fig. 1, 4c showed a maximum sharp absorption peak at approximately 308 nm accompanied by a shoulder peak at near 375 nm and these were barely affected by solvent polarity. The molar absorption coefficient (ε) of 4c underwent a slight change in different organic solvents; in MeCN it had the highest value.
| Compounds | λabsa (nm) | λema (nm) | ε (M−1 cm−1) | ΦFa | SSb (nm) |
|---|---|---|---|---|---|
| a Measurements were performed in DMSO solvent. Fluorescence quantum yields were measured with quinine sulfate (ΦF = 54.6% in 0.05 M H2SO4) as the reference.b Stokes shift. | |||||
| 4a | 368 | 440 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.41 | 72 |
| 4b | 373 | 441 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
0.014 | 68 |
| 4c | 361 | 441 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
0.26 | 80 |
| 4d | 373 | 443 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
0.60 | 70 |
| 4e | 366 | 441 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
0.83 | 75 |
Obviously, 4c shows the fluorescence emission wavelength at 438 nm with 375 nm as the excitation wavelength in different organic solvents, except for a slight blue shift in CH3CN (410 nm). It's worth noting that the fluorescence intensity of 4c is much higher in DMSO than other solvents and thus we can anticipate high fluorescence quantum yields in DMSO. The Stokes shift is 63 nm in DMSO. Therefore, we selected DMSO as the best solvent for studies on the fluorescent properties of compound 4.
The absorption and fluorescence emission spectra of compounds 4a–4e in DMSO are detailed in Table 1 and Fig. 2. They show similar absorption, but have a slight change in intensity with different substituents on the phenyl ring. Similarly, the fluorescence emission wavelength of 4 was at about 440 nm and showed a strong blue fluorescence, but fluorescence intensity was significantly different with the different groups substituted on the phenyl ring of the coumarin-fused dihydropyridine derivatives of 4. As shown in Fig. 2, compound 4e, with p-methyl substituted on the phenyl ring, had the maximum fluorescence intensity and also the fluorescence quantum yield reached 0.83. Compounds 4a (H), 4c (4-OMe), and 4d (3-Cl) all had strong blue fluorescence and the fluorescence quantum yields were respectively, 0.41, 0.26, and 0.60. It is worth noting that compound 4e shows an unexpected higher fluorescence quantum yield (ΦF = 0.83) than most of the fluorophores, and the Stokes shift of 4e is 75 nm. Compound 4b, with p-nitro substituted on the phenyl ring, displayed the maximum absorption and the minimum fluorescence intensity, so it's fluorescence is weak and the fluorescence quantum yield is only 0.014. In addition, through the TD-DFT calculation, the HOMO–LUMO energy gap of compound 4b is largest, which makes the transition hard to occur. As shown in Table 1, the Stokes shift of compounds 4a, 4b, 4c, 4e, and 4d, respectively were 72, 68, 80, 70, and 75 nm in DMSO.
 | ||
| Fig. 2 Left: Absorption spectra of 4a–4e (1.0 × 10−5 mol L−1) in DMSO (PMT voltage: 900 V). Right: Fluorescence emission spectra of 4a–4e in DMSO (PMT voltage: 600 V). | ||
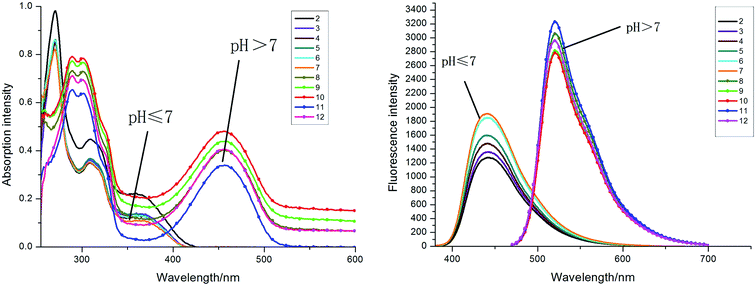
On the other hand, we also examined absorption and fluorescence properties of compound 4 under different pH from 2 to 12 in a DMSO–water mixture (DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 9
H2O = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). As shown in Fig. 3 and 4, compound 4a exhibited different properties in UV-Vis absorption and fluorescence spectra. A new absorption peak appeared at 455 nm, which is situated in the visible region, as the pH value gradually increased from 7 to 12 in a DMSO–water mixture. The solution was colorless under acidic conditions and turned yellow under basic conditions. The fluorescence intensity of 4a was obviously larger under alkaline conditions than under acidic conditions. The maximal emission wavelength of 4a had an obvious red shift (103 nm) under different pH conditions; it displayed blue fluorescence at 450 nm under acidic conditions (pH: 2–7) and yellow-green fluorescence at 553 nm under basic conditions (pH: 7–12). This effect causes significant changes in fluorescence color; blue in acid solution and yellow-green in an alkaline environment (Fig. 4). Moreover, the substituted compounds 4c–4e exhibited the same property accompanied by the change of pH value, and the maximal absorption wavelength displayed a red shift from 375 nm to 450 nm when the solution changed from acid to alkaline. The fluorescence intensity varied with the pH and the fluorescence intensity reached a maximum when the pH value of the solution was pH 11. However, the fluorescence emission wavelength of compound 4b did not change significantly in alkaline condition; rather it happened due to an intermolecular charger transfer (ICT) process (Fig. 5).27
1). As shown in Fig. 3 and 4, compound 4a exhibited different properties in UV-Vis absorption and fluorescence spectra. A new absorption peak appeared at 455 nm, which is situated in the visible region, as the pH value gradually increased from 7 to 12 in a DMSO–water mixture. The solution was colorless under acidic conditions and turned yellow under basic conditions. The fluorescence intensity of 4a was obviously larger under alkaline conditions than under acidic conditions. The maximal emission wavelength of 4a had an obvious red shift (103 nm) under different pH conditions; it displayed blue fluorescence at 450 nm under acidic conditions (pH: 2–7) and yellow-green fluorescence at 553 nm under basic conditions (pH: 7–12). This effect causes significant changes in fluorescence color; blue in acid solution and yellow-green in an alkaline environment (Fig. 4). Moreover, the substituted compounds 4c–4e exhibited the same property accompanied by the change of pH value, and the maximal absorption wavelength displayed a red shift from 375 nm to 450 nm when the solution changed from acid to alkaline. The fluorescence intensity varied with the pH and the fluorescence intensity reached a maximum when the pH value of the solution was pH 11. However, the fluorescence emission wavelength of compound 4b did not change significantly in alkaline condition; rather it happened due to an intermolecular charger transfer (ICT) process (Fig. 5).27
TD-DFT calculations were performed during this study to further understand the mechanism of the fluorescence intensity with different groups from the energy and charge transfer model (see Fig. 7). In addition, we calculated the fluorescence emission spectra and the HOMO–LUMO energy gap30 by the TD-DFT method as implemented (see Table 2). From this calculation, compound 4b requires the most energy (ΔE = 0.66 eV) to finish the HOMO–LUMO transition and compound 4e requires the least energy (ΔE = 0.31 eV) to finish HOMO–LUMO transition. Therefore, the photochemical property of compound 4b (ΦF = 0.014) is worse than compound 4e (ΦF = 0.83).
| Compounds | λcala (nm) | λexpb (nm) | λcalc (nm) | λexpd (nm) | fe | Transition character | Energy gap (eV) |
|---|---|---|---|---|---|---|---|
| a Theoretical calculations of basis sets B3LYP/6-311G without solution (the calculated emission maximums).b λmax in DMSO solvent (the experimental emission maximums).c Theoretical calculations of basis sets B3LYP/6-311G without solution (the calculated absorption maximums).d λmax in DMSO solvent (the experimental absorption maximums).e Oscillator strength coefficients. | |||||||
| 4a | 450 | 440 | 372 | 368 | 0.0122 | HOMO → LUMO | 0.44 |
| 4b | 473 | 441 | 375 | 373 | 0.0093 | HOMO → LUMO | 0.66 |
| 4c | 455 | 441 | 368 | 361 | 0.0154 | HOMO → LUMO | 0.44 |
| 4d | 447 | 443 | 373 | 373 | 0.0189 | HOMO → LUMO | 0.39 |
| 4e | 459 | 441 | 367 | 366 | 0.0215 | HOMO → LUMO | 0.31 |
Conclusions
The coumarin fused dihydropyridine derivatives were prepared by an efficient one-pot, three-component reaction and obtained in good yield. Optical properties detections indicated that these coumarin derivatives had excellent fluorescence with high fluorescent quantum yields and exhibited significant emission changes in their fluorescence spectra with changes of pH values. TD-DFT calculations were performed with this study to support our experiment results. Findings will be useful for design of new pH probe fluorescent dyes with high fluorescent quantum yields and chemical sensors for detecting pH environments.Acknowledgements
We gratefully acknowledge the financial supported by the Natural Science Foundation of China (21176223) and the National Natural Science Foundation of Zhejiang (LY13B020016) and the Key Innovation Team of Science and Technology in Zhejiang Province (2010R50018).References
- L. L. Long, X. F. Li, D. D. Zhang, S. C. Meng, J. F. Zhang, X. L. Sun, C. Zhang, L. P. Zhou and L. Wang, RSC Adv., 2013, 3, 12204 RSC.
- A. Thakur, R. Singla and V. Jaitak, Eur J Med Chem, 2015, 101, 479 CrossRef PubMed.
- J. Zheng, F. Huang, Y. J. Li, T. W. Xu, H. Xu, J. H. Jia, Q. Ye and J. R. Gao, Dyes Pigm., 2015, 113, 502 CrossRef CAS.
- U. P. Raghavendra, M. Basanagouda and J. Thipperudrappa, Spectrochim. Acta, Part A, 2015, 150, 350 CrossRef CAS PubMed.
- G. Li, J. Wang, X. Li, L. Cao, N. Lv, G. Chen, J. Zhu and J. Si, Phytochem. Lett., 2015, 13, 123 CrossRef CAS.
- Z. M. Hudson, X.-Y. Liu and S. Wang, Org. Lett., 2011, 13, 300 CrossRef CAS PubMed.
- N. Boens, V. Leen, W. Dehaen, L. N. Wang, K. Robeyns, W. W. Qin, X. L. Tang, D. Beljonne, C. Tonnele, J. M. Paredes, M. J. Ruedas-Rama, A. Orte, L. Crovetto, E. M. Talavera and J. M. Alvarez-Pez, J. Phys. Chem. A, 2012, 116, 9621 CrossRef CAS PubMed.
- Y. Kubota, Y. Ozaki, K. Funabiki and M. Matsui, J. Org. Chem., 2013, 78, 7058 CrossRef CAS PubMed.
- L. Skowronski, O. Krupka, V. Smokal, A. Grabowski, M. Naparty and B. Derkowska-Zielinska, Opt. Mater., 2015, 47, 18 CrossRef CAS.
- Y. Zhang, X. Guo, X. Tian, A. Liu and L. Jia, Sens. Actuators, B, 2015, 218, 37 CrossRef CAS.
- V. Lakshmi and M. Ravikanth, J. Org. Chem., 2011, 76, 8466 CrossRef CAS PubMed.
- S. Chen, F. Jiang, Z. Cao, G. Wang and Z.-M. Dang, Chem. Commun., 2015, 51, 12633 RSC.
- Faridoon, T. O. Olomola, M. Tukulula, R. Klein and P. T. Kaye, Tetrahedron, 2015, 71, 4868 CrossRef CAS.
- G. Mata and N. W. Luedtke, Org. Lett., 2013, 15, 2462 CrossRef CAS PubMed.
- M. C. R. Castro, P. Schellenberg, M. Belsley, A. M. C. Fonseca, S. S. M. Fernandes and M. M. M. Raposo, Dyes Pigm., 2012, 95, 392 CrossRef CAS.
- T. Z. Yu, S. D. Yang, Y. L. Zhao, H. Zhang, X. Q. Han, D. W. Fan, Y. Q. Qiu and L. L. Chen, J. Photochem. Photobiol., A, 2010, 214, 92 CrossRef CAS.
- H. X. Xu, X. Q. Wang, C. L. Zhang, Y. P. Wu and Z. P. Liu, Inorg. Chem. Commun., 2013, 34, 8 CrossRef CAS.
- D. Li, W. Zhao, X. Sun, J. Zhang, M. Anpo and J. Zhao, Dyes Pigm., 2006, 68, 33 CrossRef CAS.
- T. Yan, J. Chen, S. Wu, Z. Mao and Z. Liu, Org. Lett., 2014, 16, 3296 CrossRef CAS PubMed.
- S. Barman, S. K. Mukhopadhyay, M. Gangopadhyay, S. Biswas, S. Dey and N. D. P. Singh, J. Mater. Chem. B, 2015, 3, 3490 RSC.
- L. Peng, G. Zhang, D. Zhang, J. Xiang, R. Zhao, Y. Wang and D. Zhu, Org. Lett., 2009, 11, 4014 CrossRef CAS PubMed.
- X. D. Liu, Y. Xu, R. Sun, Y. J. Xu, J. M. Lu and J. F. Ge, Analyst, 2013, 138, 6542 RSC.
- M. Q. Liu, M. S. Hu, Q. Jiang, Z. Y. Lu, Y. Huang, Y. F. Tan and Q. Jiang, RSC Adv., 2015, 5, 15778 RSC.
- E. Oliveira, C. I. M. Santos, H. M. Santos and A. Fernandez-Lodeiro, Dyes Pigm., 2014, 110, 219 CrossRef CAS.
- N. Ahmed, B. V. Babu, S. Singh and P. M. Mitrasinovic, Heterocycles, 2012, 85, 1629 CrossRef CAS.
- L. J. Xie, Y. H. Chen, W. T. Wu, H. M. Guo, J. Z. Zhao and X. R. Yu, Dyes Pigm., 2012, 92, 1361 CrossRef CAS.
- T. H. Nguyen, T. Venugopala, S. Y. Chen, T. Sun, K. T. V. Grattan, S. E. Taylor, P. A. M. Basheer and A. E. Long, Sens. Actuators, B, 2014, 191, 498 CrossRef CAS.
- D. Wang, L.-J. Chen, J.-L. Liu, X.-Y. Wang, Y.-L. Wu, M.-J. Fang, Z. Wu and Y.-K. Qiu, J. Chromatogr. A, 2015, 1406, 215 CrossRef CAS PubMed.
- X. B. Huang, Y. Dong, Q. W. Huang and Y. X. Cheng, Tetrahedron Lett., 2013, 54, 3822 CrossRef CAS.
- Y. Shan, Y. Sun, N. Sun, R. Guan, D. Cao, K. Wang, Q. Wu and Y. Xu, Inorg. Chem. Commun., 2015, 59, 68 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra05996a |
| This journal is © The Royal Society of Chemistry 2016 |