Facile one pot synthesis of mesoporous organic–inorganic hybrid aluminosilicate spheres with ultra-high aluminium contents and their enhanced adsorption behavior for methylene blue†
Shangjing Zeng,
Runwei Wang*,
Zongtao Zhang and
Shilun Qiu
State Key Lab of Inorganic Synthesis and Preparative Chemistry, Jilin University, Changchun 130012, China. E-mail: rwwang@jlu.edu.cn
First published on 6th May 2016
Abstract
Hybrid mesoporous organo-aluminosilicate spheres (HMAS) with ultra-high aluminium contents have been successfully synthesized with co-condensation of sodium aluminate and organosilane at room temperature using cetyltrimethyl-ammonium bromide (CTAB) as a templating agent. The hybrid framework remains intact after template removal with mild acidity solutions, and the template-extracted samples have a high content of framework aluminium and textural porosity. Additionally, the high Al-containing hybrid mesoporous materials exhibit excellent removal performance for methylene blue (397 mg g−1) owing to their extremely low Si/Al ratio and high surface area.
Organic dyes are increasingly used in realms such as textile, paper, dyeing, printing, cosmetics and so on. However, most of them and their degradation products are toxic and carcinogenic.1 Environmental problems will be produced if dye waste-effluents are discharged directly into the environment. A variety of technologies have been developed to remove organic pollutants, and the adsorption technique has been proven to be one of the best approaches of dye removing from wastewaters due to its advantages of high efficiency, low cost and availability of various adsorbents.2–6 Recently, many adsorbents including active carbon, zeolites, mesoporous silicas, nanostructured TiO2 and mesoporous alumina are available for removing organic dyes.7–10 Among them, activated carbon is the most extensively used adsorbent, but it was still considered expensive and thus restricted it to board application. Therefore, many researchers have focus the efforts on novel adsorbents, which pose a large adsorption capacity, fast adsorption rate, low cost, and should be easy to separate from solution.11–17
Recently, periodic mesoporous organosilicas (PMO) have been discovered and functionalized through coupling of silica-based inorganic and organic components by template synthesis.18–24 The hybrid materials have attracted considerable interests owing to a wide range of organic bridges incorporated in the mesoporous walls.25 Nevertheless, a few methods for the preparation of materials with mixed oxide and organic hybrid walls have been reported, which would hinder their use for various potential applications. The heteroatom substituted PMOs might offer significant advantages over the traditional silica-based materials due to the increased control of hydrophobicity/hydrophilicity of the surface of the pores.26 Moreover, metal-substituted PMOs are usually more active and selective than their silica counterparts. The incorporation of heteroatoms into hybrid mesoporous organosilicas offers the opportunity for applications in catalysis and separation.27–29
It is well known that incorporation of the aluminium content in the silica-based mesoporous materials should be one of the key factors for their applications as adsorbents and catalysts. Al-MCM-41 displays the higher organic dye adsorption capacities than pure siliceous MCM-41.30 Similarly, Al-SBA-15 materials shows a greater adsorption capacity to methylene blue than SBA-15.31 However, it is still a great challenge to improve the adsorption capacity of them by introducing the metal species into its structure. The flexibility of Al-PMO offers new opportunities of the meso-structured materials for multi-functional catalysts and adsorbents.28 Al-containing PMO (Al-PMO) materials have been reported to show interesting catalytic properties.29 The incorporation of heteroatoms will expand the range both of adsorption and catalytic reactions for their application. However, the aluminium content incorporated in these mesoporous organosilicas seems to be limited. Thus, how to use a flexible method for synthesis of aluminium-rich inorganic–organic hybrid mesoporous materials with designed chemical components and controlled morphologies is a huge challenge.
In this paper, we prepared hybrid mesoporous aluminosilicate spheres (HMAS) with a Si/Al ratio as low as 1.74 under static conditions via a cationic surfactant pathway at room temperature, and the aluminium is exclusively tetrahedral-coordinated dispersed in ethane–silica frameworks. In addition, adsorption of dyes on Al-containing hybrid materials from aqueous solution was investigated.
Fig. 1 shows the representative SEM micrographs of the hybrid mesoporous aluminosilicates with different amounts of aluminium in the synthesis mixture (Si/Al = 0.6–74). It can be seen that the sample HMAS1 and HMAS2 consist entirely of spherical particles with size ranging from 1 to 3 μm for micro-spheres. Additionally, the surface of these mesoporous spheres in the product are very smooth rather than faceted or corrugated. Nevertheless, the amounts of aluminium incorporated into the mesoporous framework exhibit a significant influence on the size and morphology of the synthesized samples, the spherical particles will be emerged with increasing aluminium contents in the framework of Al-containing hybrid material, that is the Si/Al ratio is less than or equal to 2 (Fig. 1a and b), which is consisted with Zhao et al. reported.32,33 Likewise, we noted that HMAS4 exhibited elliptical shapes with a size of ca. 2 μm when the Si/Al ratio increased to 4 from 2 (Fig. 1a). Furthermore, HMAS0.67 leads to very large spherical particles (2–8 μm) with further increasing aluminium content (Fig. 1d). Additionally, stirring effects on morphologies were investigated. A few larger irregular monoliths around spheres would emerge under stirring condition (Fig. S1†), which suggested stirring is unfavorable for the formation of spherical morphology. This phenomenon conforms to Qi's study.34
 | ||
| Fig. 1 SEM images of mesoporous organo-aluminosilicate spheres with diverse Al content (a) HMAS4, (b) HMAS2, (c) HMAS1, (d) HMAS0.67. | ||
Fig. 2 shows the XRD patterns of the mesoporous organo-aluminosilicate spheres. Remarkably, the sample HMAS4 exhibits characteristic peaks identical to that observed for materials of typical ordered MCM-41 (Fig. 2A(a)). However, with gradually decreasing Si/Al ratios, other samples HMASn (n = 0.67, 1, 2) give only one broad diffraction peak (100) with decreased intensity and the main peak shifts into low angle diffraction region (Fig. 2A(b–d)), which indicate that no long range ordering structure obtained. The XRD patterns of surfactant-free samples are given in Fig. 2B, in this case, we used two different procedures for the removal of organic surfactant, one is HCl–EtOH mixture for sample HMAS4, the other is NH4NO3–EtOH mixture for higher aluminium samples (HMAS2, HMAS1, HMAS0.67).35 The XRD patterns of HMASn (n = 0.67, 1, 2) after template removal with mild acidity solutions resemble that of the untreated ones, this is indicative of hybrid structures remain largely unaffected during the template extraction process. Moreover, these samples show the same d100 reflection angle as the as-synthesized sample, indicating less lattice contraction after the organic template removal process.35 However, the diffraction peak of the sample HMAS4 extracted by lower concentration of HCl in ethanol is board with strongly decreased intensity (Fig. 2B(a)), indicating its structure partially ruined.26
 | ||
| Fig. 2 XRD patterns of as-synthesized (A) and template-free (B) hybrid mesoporous aluminosilicates with various Al contents (a) HMAS4, (b) HMAS2, (c) HMAS1, (d) HMAS0.67. (a) Template removal in HMAS4 by extraction with an HCl–EtOH mixture.28 | ||
Very interestingly, the particle size of HMAS1 can be easily tailored by adjusting the concentration. Fig. 3 shows the SEM and TEM images of the small HMAS1 prepared by diluting the initial concentration of the solution to tenfold. The prepared organo-aluminosilicate particles exhibit monodispersed spherical morphology and uniform particle size with the average sizes of ca. 1 μm. TEM images of the sample demonstrated a clear arrangement of pores with uniform size (Fig. 3c). Notably, the surface of spheres is rather smooth. In addition, we noted that the water amount must be restrained in a certain range so as to keep the surface smooth (Fig. S2†).
Fig. 4 shows the 29Si and 27Al MAS NMR spectra of the template-extracted sample HMAS1, the 29Si NMR spectrum demonstrates a broad peak at −53.6 ppm for the T2 site (SiC(OH)(OSi)2) or the T3 site (SiC(OAl)(OSi)2), while the shoulder at −44.7 ppm is probably attributed to the T1 silicon site (SiC(OH)2(OSi))36–38 (Fig. 4a). Surprisingly, there is no obvious T3 site (SiC(OSi)3, ca. −65 ppm), this is indicative of methyl silicon atoms are within the mainly condensed T2 environments. When compared to the spectra for Al-MCM-41 and mesoporous Al-PMOs, some similarities are observed.26,39 Therefore, to some extent, the enhanced resonance T2 indicates the sample HMAS1 is aluminium-rich material, which agree to the reported literature.26,40 Moreover, the absence of Qn sites (−90 to −120 ppm) in the spectra suggests no Si–C bond cleavage during either the synthesis or surfactant extraction periods. In addition, 29Si NMR spectrum of the sample HMAS0.67 is identical to that of HMAS1 (Fig. S3(b)†). 27Al MAS NMR spectrum exhibits a single resonances attributable to the tetrahedral aluminium environment (Fig. 4(b)), indicating aluminium is tetrahedrally incorporated exclusively into the hybrid framework. However, the higher aluminium-containing sample HMAS0.67 (Fig. S3(b)†) exhibits two resonances at 55 ppm and 0 ppm, indicating the presence of both extra-framework (Al(6)) and framework aluminium (Al(4)). The weak resonance at 0 ppm is due to the aluminium species in octahedral coordination, which is usually assigned to the extra-framework aluminium centers. From the Al(4)/Al(6) ratio, we can see that approximately 96 mol% of aluminium is tetrahedrally coordinated in the framework of the mesoporous aluminosilicate material.26,28 Additionally, the Si/Al ratios determined by ICP indicate a considerable amount of aluminium remains in the framework (Table 1).
The FT-IR spectra further confirm the successful synthesis of HMAS spheres in Fig. S5.† The Si–O vibrations of Si–OH and Si–O–Si groups are observed in the range of 850 to 1100 cm−1. Both HMAS samples exhibit bands at 1274 and 1406 cm−1, corresponding to C–H deformation vibrations, while the peak at 695 and 768 cm−1 is assigned to Si–C stretching vibrations.41 Furthermore, absorption bands at 2811, 2898 cm−1 which could be assigned to the CTAB can only be seen in the spectra of the as-synthesized sample indicating that the surfactant is almost completely removed by extraction. The N2 sorption isotherms of HMAS are presented in Fig. 5 and the corresponding textural properties are listed in Table 1. Data from the N2 adsorption–desorption analysis show that the solid synthesized from the synthetic mixtures with different Si/Al ratios have typical type IV isotherms, indicative of the existence of mesoporous structure.35 Moreover, the isotherm of HMAS0.67 displays a sharp increase in the relative pressure 0.45–0.9, implying the presence of the porous structures as a function of aluminium contents in their structure.26 The pore-size distribution curve presents a bimodal mesopore size of ca. 2.3 and 15 nm obtained from the adsorption branch by using the Barrett–Joyner–Halenda (BJH) model (Fig. 5B). Furthermore, typical TEM image of the sample HMAS0.67 shows some irregular debris with mesoporous structures are around the spheres, and the HRTEM image reveals the bimodal porous structure of HMAS0.67 originating from the larger voids between the irregular debris and the smaller worm-like mesoporous channels of the aluminosilicate sphere, which should be responsible for the pore sizes of 15 and 2.3 nm shown in Fig. S4,† respectively. The BET surface areas and pore volumes of HMAS were in the range of 535–721 m2 g−1 and 0.29–0.81 m3 g−1, respectively.
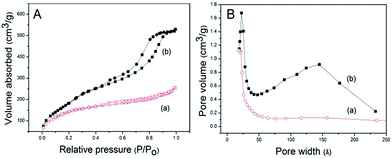 | ||
| Fig. 5 Nitrogen sorption isotherms (A) and the corresponding pore size distribution (B) of the HMAS samples (a) HMAS1, (b) HMAS0.67. | ||
Removal of dyes discharged into wastewater, such as methylene blue (MB), is becoming an intense issue due to their high toxicity and carcinogenicity to the environment.42 To investigate the advantages of the aluminium-rich hybrid mesoporous aluminosilicate spheres (HMAS), the dye adsorption using MB is studied to determine the adsorption abilities of hybrid mesoporous materials. UV/Vis absorption spectroscopy was employed to monitor the adsorption process of MB. The adsorption isotherms of MB on HMAS1 and MCM-41 were first obtained by plotting the equilibrium adsorption capacity (Qe, mg g−1) vs. the equilibrium solution concentration of MB (Ce, mg L−1). Fig. 6A shows the adsorption isotherms of MB on HMAS1 and silica-based mesoporous material MCM-41. Remarkably, HMAS1 has a large adsorption capacity for MB over silica-based material. The maximum adsorption capacity (Qmax) of HMAS1 to MB is 397 mg g−1, which is much higher than that of MCM-41 (81.3 mg g−1), and much higher than those reported value on conventional silica-based mesoporous materials (Table S1†). It is supposed that such an extraordinary adsorption performance resulted from the nature of hydrophobic mesoporous organosilicas and numerous efficient adsorption sites related to high aluminium contents.24,43 The adsorption isotherm constants for HMAS1 and MCM-41 were listed in Table 2. It is obvious that correlation coefficient fit the Langmuir adsorption isotherm well, indicating the possibility of the monolayer adsorption of MB onto the adsorbents. Fig. 6B displays the dynamic adsorption curves of MB on HMAS1 and MCM-41 at 25 °C. As seen from the curves, the adsorption capacity of both adsorbents increased rapidly within 25 min (removal efficiency: 96% for HMAS1, 75% for MCM-41) and changes slightly until 40 min, after that no further adsorption occurs along with the increase of contact time. It also can be directly observed that the colors of the solutions containing dyes became pale after 40 min (Fig. S6†). This clearly indicates significantly accelerated adsorption kinetics for MB removal from aqueous solution by hybrid mesoporous aluminosilicate materials. It is probably coupling the effect of electrostatic, hydrophobic interaction and heteroatoms bond mechanisms in the adsorption process.44 It is suggested that the hybrid mesoporous aluminosilicate microsphere has a great potential application and could be considered as an alternative efficient adsorbent for dye removal in wastewater treatment due to its enhanced adsorption performance.
| Adsorbents | Qm (mg g−1) | KL (L mg−1) | R2 |
|---|---|---|---|
| HMAS1 | 397 | 0.735 | 0.999 |
| MCM-41 | 81.3 | 0.984 | 0.999 |
Conclusion
Mesoporous organo-aluminosilicate spheres with high aluminium contents have been synthesized through a facile procedure under static conditions at room temperature. The HMAS exhibit mainly tetrahedral coordination of aluminium after template removal with mild acidity solutions, which can avoid both the destruction of hybrid structure and dealumination. Moreover, the particle size of the HMAS can be easily tailored by controlling the concentration of the solution. The HMAS exhibits excellent removal capabilities of methylene (397 mg g−1), which are higher than conventional silica-based mesoporous such as MCM-41. Thus, hybrid organosilica microspheres with ultra-high aluminium contents may provide a new chance for the application of periodic mesoporous organosilicas for adsorption and catalysis.Notes and references
- M. T. Yagub, T. K. Sen, S. Afroze and H. M. Ang, Adv. Colloid Interface Sci., 2014, 209, 172 CrossRef CAS PubMed.
- L. C. Juang, C. C. Wang and C. K. Lee, Chemosphere, 2006, 64, 1920 CrossRef CAS PubMed.
- C. K. Lee, S. S. Liu, L. C. Juang, C. C. Wang, K. S. Lin and M. D. Lyuk, J. Hazard. Mater., 2007, 147, 997 CrossRef CAS PubMed.
- S. B. Wang and H. Li, Microporous Mesoporous Mater., 2006, 97, 21 CrossRef CAS.
- L. Q. Yang, Y. F. Li, H. Y. Hu, X. L. Jin, Z. F. Ye, Y. X. Ma and S. D. Zhang, Chem. Eng. J., 2011, 173, 446 CrossRef CAS.
- C. X. Gui, Q. J. Li, L. L. Lv, J. Qu, Q. Q. Wang, S. M. Hao and Z. Z. Yu, RSC Adv., 2015, 5, 20440 RSC.
- J. R. Deka, Y. H. Lin and H. M. Kao, RSC Adv., 2014, 4, 49061 RSC.
- K. Y. Ho, G. M. Kay and K. L. Yeung, Langmuir, 2003, 19, 3019 CrossRef CAS.
- R. Wang, X. Cai and F. L. Shen, Appl. Surf. Sci., 2014, 305, 352 CrossRef CAS.
- P. V. Vigon, M. Sevilla and A. B. Fuertes, Microporous Mesoporous Mater., 2013, 176, 78 CrossRef.
- Q. Qu and Z. Gu, Anal. Methods, 2014, 6, 1397 RSC.
- N. Bayal and P. Jeevanandam, J. Nanopart. Res., 2013, 15, 2006 CrossRef.
- W. C. Chang, J. R. Deka, H. Y. Wu, F. K. Shieh, S. Y. Huang and H. M. Kao, Appl. Catal., B, 2013, 142–143, 817 CrossRef CAS.
- Z. Yan, S. Y. Tao, J. X. Yin and G. T. Li, J. Mater. Chem., 2006, 16, 23470 Search PubMed.
- Y. Q. Gan, N. Tian, X. Tian, L. Ma, W. Wang, C. Yang, Z. Zhou and Y. Wang, J. Porous Mater., 2015, 22, 147 CrossRef CAS.
- S. B. Wang and H. T. Li, Microporous Mesoporous Mater., 2006, 97, 21 CrossRef CAS.
- Y. Liu, Y. Zheng and A. Q. Wang, J. Environ. Sci., 2010, 22, 486 CrossRef CAS.
- F. Hoffmann, M. Cornelius, J. Morell and M. Froba, Angew. Chem., Int. Ed., 2006, 45, 3216 CrossRef CAS PubMed.
- A. Sayari and S. Hamoudi, Chem. Mater., 2001, 13, 3151 CrossRef CAS.
- Y. F. Hu, K. Qian, P. Yuan, Y. Wang and C. Yu, Mater. Lett., 2011, 65, 21 CrossRef CAS.
- S. S. Yoon, W. J. Son, K. Biswas and W. S. Ahn, Bull. Korean Chem. Soc., 2008, 29, 609 CrossRef CAS.
- S. Y. Guan, S. Inagaki, T. Ohsuna and O. Terasaki, J. Am. Chem. Soc., 2000, 122, 5660 CrossRef CAS.
- S. Inagaki, S. Guan, Y. Fukushima, T. Ohsuna and O. Terasaki, J. Am. Chem. Soc., 1999, 121, 9611 CrossRef CAS.
- S. O. Ganiyu, C. Bispo, N. Bion, P. Ferreira and I. B. Gener, Micro. Meso. Mater., 2014, 200, 117 CrossRef CAS.
- E. R. Magdaluyo Jr, R. V. R. Virtudazo, L. P. Cruz, E. V. Castriciones and H. D. Mendoza, Philipp. J. Sci., 2009, 138, 5 Search PubMed.
- B. J. Hughes, J. B. Guibaud, M. Allix and Y. Z. Khimyak, J. Mater. Chem., 2005, 15, 4728 RSC.
- Y. Xia, W. Wang and R. Mokaya, J. Am. Chem. Soc., 2005, 127, 790 CrossRef CAS PubMed.
- Q. H. Yang, J. Yang, Z. Feng and Y. Li, J. Mater. Chem., 2005, 15, 4268 RSC.
- Q. H. Yang, J. Liu, L. Zhang and C. Li, J. Mater. Chem., 2009, 19, 1945 RSC.
- S. Eftekhari, A. H. Yangjeh and S. Sohrabnezhad, J. Hazard. Mater., 2010, 178, 349 CrossRef CAS PubMed.
- Z. Y. Wu, Q. Lu, W. H. Fu, S. Wang, C. Liu, N. Xu, D. Wang, Y. M. Wang and Z. Chen, New J. Chem., 2015, 39, 985 RSC.
- W. P. Guo and X. S. Zhao, Microporous Mesoporous Mater., 2005, 85, 32 CrossRef CAS.
- Y. D. Xia, W. X. Wang and R. Mokaya, J. Am. Chem. Soc., 2005, 127, 790 CrossRef CAS PubMed.
- L. M. Qi, J. M. Ma, H. M. Cheng and Z. G. Zhao, Chem. Mater., 1998, 10, 1623 CrossRef CAS.
- S. Shylesh, P. P. Samuel and A. P. Singh, Microporous Mesoporous Mater., 2007, 100, 250 CrossRef CAS.
- K. Yamamoto, Y. Sakata, Y. Nohara, Y. Takahashi and T. Tatsumi, Science, 2003, 300, 470 CrossRef CAS PubMed.
- J. Abia, Chromatography, 2015, 2, 141 CrossRef.
- L. Fu, R. A. S. Ferreira, N. J. O. Silva, A. J. Fernades, P. R. Claro, I. S. Goncalves, V. Z. Bermudez and L. D. Carlos, J. Mater. Chem., 2005, 15, 3117 RSC.
- Z. H. Luan, C. F. Cheng, W. Z. Zhou and J. Klinowski, J. Phys. Chem., 1995, 99, 1018 CrossRef CAS.
- G. Bellussi, A. Carati, E. D. Paola, R. Millini, W. O. Parker Jr, C. Rizzo and S. Zanardi, Microporous Mesoporous Mater., 2008, 113, 252 CrossRef CAS.
- O. Muth, C. Schellbach and M. Froba, Chem. Commun., 2011, 2032 Search PubMed.
- M. Rafatullah, O. Sulaiman, R. Hashim and A. Ahmad, J. Hazard. Mater., 2010, 177, 70 CrossRef CAS PubMed.
- M. A. Zanjanchi, A. Ebrahimian and Z. Alimohammadi, Opt. Mater., 2007, 29, 794 CrossRef CAS.
- C. Zhou, Q. Gao, W. Luo, Q. Zhou, H. Wang, C. Yan and P. Duan, J. Taiwan Inst. Chem. Eng., 2015, 52, 147 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra05922h |
| This journal is © The Royal Society of Chemistry 2016 |



