TiO2 nanoparticle interactions with supported lipid membranes – an example of removal of membrane patches†
Abstract
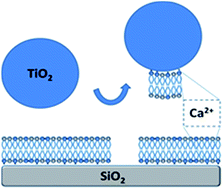
There is a need for different levels of model systems for effect studies of engineered nanoparticles and the development of nanoparticle structure–activity relationships in biological systems. Descriptors for nanoparticles based on their interactions in molecular model systems may become useful to predict toxicological responses of the nanoparticles in cells. Towards this end, we report on nanoparticle-induced formation of holes in supported model membranes. Specifically, TiO2 nanoparticle – lipid membrane interactions were studied under low ionic strength, basic conditions (pH 8), using different membrane compositions and several surface-sensitive analytical techniques. It was found that for mixed POPC/POPG (PG fractions ≥ 35%) membranes on silica supports, under conditions where electrostatic repulsion was expected, the addition of TiO2 nanoparticles resulted in transient interaction curves, consistent with the removal of part of the lipid membrane. The formation of holes was inferred from quartz crystal microbalance with dissipation (QCM-D) monitoring, as well as from optical measurements by reflectometry, and also verified by atomic force microscopy (AFM) imaging. The interaction between the TiO2 nanoparticles and the PG-containing membranes was dependent on the presence of Ca2+ ions. A mechanism is suggested where TiO2 nanoparticles act as scavengers of Ca2+ ions associated with the supported membrane, leading to weakening of the interaction between the membrane and the support and subsequent removal of lipid mass as TiO2 nanoparticles spontaneously leave the surface. This mechanism is consistent with the observed formation of holes in the supported lipid membranes.


 Please wait while we load your content...
Please wait while we load your content...