Lithium–air battery cathode modification via an unconventional thermal method employing borax†
Andy Fiedlera,
Andrew P. Vogt‡
bc,
Lukas Pfaffmanna,
Vanessa Trouilleta,
Jörg T. Breukelgend,
Ralf Köppee,
Christopher Barner-Kowollikbc,
Helmut Ehrenberga and
Frieder Scheiba*a
aInstitute for Applied Materials (IAM-ESS), Karlsruhe Institute of Technology (KIT), Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany. E-mail: frieder.scheiba@kit.edu; Fax: +49 721 608 28521; Tel: +49 721 608 28520
bPreparative Macromolecular Chemistry, Institut für Technische Chemie und Polymerchemie, Karlsruhe Institute of Technology (KIT), Engesserstr. 18, 76128 Karlsruhe, Germany
cInstitut für Biologische Grenzflächen (IBG), Karlsruhe Institute of Technology (KIT), Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany
dStaatliche Majolika Manufaktur Karlsruhe GmbH, Ahaweg 6-8, 76131 Karlsruhe, Germany. E-mail: J.Breukelgen@majolika-karlsruhe.com; Fax: +49 721 912 3778; Tel: +49 721 912 3770
eInstitut für Anorganische Chemie, Karlsruhe Institute of Technology (KIT), Engesserstr. 15, 76131 Karlsruhe, Germany. E-mail: ralf.koeppe@kit.edu; Fax: +49 721 608 44854; Tel: +49 721 608 43086
First published on 5th July 2016
Abstract
A novel, unconventional thermal treatment employing borax for preparing porous carbon materials is presented. The new method was used to prepare carbon felt electrodes for use in lithium–air batteries. The etching of the carbon fiber surface was found to be highly controllable by the amount of borax. The resulting felts were characterized by cyclic voltammetry (CV), secondary electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDX), and X-ray photoelectron spectroscopy (XPS). The borax treatment resulted in a change of the size, shape and orientation of the Li2O2 crystals formed during discharge.
Lithium–air batteries, functioning on the premise of the reversible reaction 2Li + O2 ⇌ Li2O2, have received much attention due to the high theoretical energy density determined to be 2–3 kW h kg−1 on the cell level, which is five times larger than any possible Li ion battery.1 A simplistic interpretation is that this means that an electric vehicle could travel more than 5 times further on a single charge than what currently exists today because to date all electric vehicles utilize Li ion batteries. Therefore, research has recently increased to better understand and improve Li–air batteries – which has included investigations of various cathode materials,2–10 employing different soluble and indissoluble catalysts for the charge and discharge processes.11,12 For further details, refer to the extensive review articles.1,13
Herein we report a method for modifying carbon felt electrodes via an unconventional thermal method employing borax. In this process the carbon felt electrode and borax are sealed under high vacuum (approx. 1 × 10−3 mbar) and subsequently placed in a pottery kiln at rt. The kiln is then heated to 800 °C, held for 30 min and allowed to cool to rt (details of the heating and cooling process can be found in the ESI, S-3 synthesis†). There are significant findings worth mentioning: (1) placing the sealed quartz ampule into the kiln already at 800 °C produced an inhomogeneous reaction where the resulting chemistry (explained in the following paragraphs) could not equilibrate and therefore created an inhomogeneous modification to the carbon cathode. (2) Employing a large pottery kiln with thick walls afforded a slow cooling process which favored a more homogenous effect on the cathode. (3) Adding dopants other than borax, such as polymeric species and small organic molecules, resulted in a coated layer of material distributed on the cathode and resulted in a decreased electrochemical activity in all cases (refer to the ESI, Fig. S3†).
The aim of the experiment reported herein was modification of the carbon electrode surface by boron through the gas phase. Unfortunately, common boron compounds possess only a very low vapor pressure: for sodium tetraborate, Na2B4O7, or boron oxide B2O3, a decomposition pressure of boron containing species is measured only at a temperature of at least 1400 K. The vapor phase of 1200 K hot sodium metaborate NaBO2 consists of only 0.01 mbar of gaseous NaBO2.14 The composition of the gas phase over mold slags of Na2O and B2O3 at 1573 K consists of about 23 mbar NaBO2 and 0.015 mbar B2O3.15 Therefore compounds of the system Na2O/B2O3 are not able to allow a gas phase controlled coating of carbon electrodes in the temperature range up to 1100 K. In principle this problem could be overcome either by the highly technical demanding condensation of gaseous reaction partners in the framework of a chemical vapor deposition (CVD) process or by the temperature dependent volatilization of a condensed phase in the presence of a gaseous reaction partner (so-called chemical transport reaction16). Despite the wide applicability of boria-forming materials, boron oxide is very unstable in water vapor.17,18 Thus, we investigated the temperature dependence of the system B2O3 (l)/H2O (g) as a potential chemical gas phase transport reaction. To ensure controlled reaction conditions, we employed borax containing crystal water (Na2B4O7·10H2O). Upon heating under vacuum, initially the water portion evaporates, later Na2B4O7 decomposes into a liquid mixture of Na2O and 2B2O3.19 Taking above precursor, the molar ratio between B and H2O constantly remains at 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 in the case of a sealed reaction vessel (as we employed). Depending on the application environments (temperature and pressure), the formation of three boron containing gas phase species seems plausible – the thermodynamic data are known with sufficient accuracy from high temperature mass spectrometry measurements. Therefore, the thermodynamic parameters of the reactions under discussion (reaction enthalpies ΔrH0298 and entropies ΔrS0298) are all well known.20,21 The gas phase compounds HBO2, B(OH)3, H3B3O6 as well as H2O are linked to each other by the following independent chemical equilibria:
10 in the case of a sealed reaction vessel (as we employed). Depending on the application environments (temperature and pressure), the formation of three boron containing gas phase species seems plausible – the thermodynamic data are known with sufficient accuracy from high temperature mass spectrometry measurements. Therefore, the thermodynamic parameters of the reactions under discussion (reaction enthalpies ΔrH0298 and entropies ΔrS0298) are all well known.20,21 The gas phase compounds HBO2, B(OH)3, H3B3O6 as well as H2O are linked to each other by the following independent chemical equilibria:
| B2O3 (l) + H2O (g) ⇌ 2HBO2 (g), ΔrH0298 = 373.8 kJ mol−1, ΔrS0298 = 213.0 J mol−1 K−1 |
| HBO2 (g) + H2O (g) ⇌ B(OH)3 (g), ΔrH0298 = −189.8 kJ mol−1, ΔrS0298 = −133.7 J mol−1 K−1 |
| 3B(OH)3 (g) ⇌ 3H2O (g) + 2H3B3O6 (g), ΔrH0298 = −20.4 kJ mol−1, ΔrS0298 = 28.4 J mol−1 K−1 |
The composition of the gas phase in an evacuated quartz ampoule can be determined by an iterative thermochemical calculation. We take into account that the hydrogen atoms originating from the borax crystal water are distributed among the gaseous species H2O, HBO2, B(OH)3 and H3B3O6 in the chemical equilibria (“determination equation”: p(H2O from decomposition of Na2B4O7·10H2O) = p(H2O) + ½p(HBO2) + ![[/]](https://www.rsc.org/images/entities/char_e0ee.gif) p(B(OH)3) +
p(B(OH)3) + ![[/]](https://www.rsc.org/images/entities/char_e0ee.gif) p(H3B3O6)). Technically, the mathematical problem is solved the following way: four vapor pressure data sets are determined using three chemical equilibria and one “determination equation”.22 This procedure proved to be successful before for the prediction of condensed phase/gas phase high temperature reactions.23 In Fig. 1, the gas phase composition of a quartz ampoule (volume 7 mL) filled with 30 mg borax is presented. At 1100 K, the ampoule contains 1.30 bar B(OH)3 and 0.9 bar H3B3O6 with a total pressure of 9.3 bar, whereas the partial pressure of HBO2 is determined to be only 1 mbar. Based on our experience, the total pressure reached here is the highest value available in a quartz ampoule. Ampoules for which a higher total pressure is calculated exploded in every case. Upon cooling the ampoule, the chemical equilibrium favors the backward reaction to the side of liquid B2O3 so that its desired condensation on the carbon electrodes as well as on the inner walls of the quartz tube takes place.
p(H3B3O6)). Technically, the mathematical problem is solved the following way: four vapor pressure data sets are determined using three chemical equilibria and one “determination equation”.22 This procedure proved to be successful before for the prediction of condensed phase/gas phase high temperature reactions.23 In Fig. 1, the gas phase composition of a quartz ampoule (volume 7 mL) filled with 30 mg borax is presented. At 1100 K, the ampoule contains 1.30 bar B(OH)3 and 0.9 bar H3B3O6 with a total pressure of 9.3 bar, whereas the partial pressure of HBO2 is determined to be only 1 mbar. Based on our experience, the total pressure reached here is the highest value available in a quartz ampoule. Ampoules for which a higher total pressure is calculated exploded in every case. Upon cooling the ampoule, the chemical equilibrium favors the backward reaction to the side of liquid B2O3 so that its desired condensation on the carbon electrodes as well as on the inner walls of the quartz tube takes place.
 | ||
| Fig. 1 Temperature dependence of the equilibrium composition of B2O3 and H2O inside a quartz ampoule (7 ml, 30 mg borax). | ||
Safety note: before such an experiment is conducted it is highly advisable that the expected total pressure is calculated. It should not exceed 10 bar. Please meet all appropriate safety instructions (protected shield, safety glasses, and gloves!). The thermochemical calculation serves only as a rough estimate as we did not take into account the water gas reaction (H2O (g) + C (s) ⇌ H2 (g) + CO (g)) as well as the Boudouard equilibrium (2CO (g) ⇌ C (s) + CO2 (g)). This procedure is allowed as we did not detect CO2 or CO inside the ampoules by gas phase Raman spectroscopy (see ESI, S-3 synthesis†).
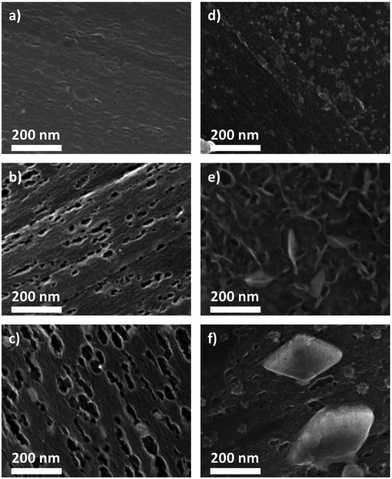
After synthesis the felt was washed in water and ethanol and dried under vacuum. Before and after synthesis the felt was analyzed via XPS. We found that the boron content on the surface increased from 1 to 5 at% from the 5 mg to the 30 mg sample (refer to the ESI, Fig. S4†). The boron is mostly in the form of B2O3, which probably forms small islands on the carbon fibers. There is no indication by XPS that boron is chemically bonded to the carbon surface. The electrochemical characterization was achieved by cyclic voltammetry. The first cycle is shown in Fig. 2. It can be observed that the onset potential of the reduction process is shifted by about 100 mV to higher potentials compared to the untreated electrode. For the sample treated with borax an increase of double layer capacity can also be observed. This is in agreement with an increase of the surface area of the carbon fibers due to the borax treatment as observed by SEM (Fig. 4).
The anodic sweep shows at least two distinct oxidation processes. This is generally attributed to a two-step oxidation process of Li2O2 and is in agreement to the hypothesis of Lu et al.24 that lithium peroxide reacts first to lithium superoxide and further to lithium and oxygen during charge.
Fig. 4 shows characteristic regions of carbon fibers treated with different borax contents (left) before and (right) after discharge. The electrochemical discharge curves are shown in Fig. 3. The surface of the fiber with 5 mg borax is slightly roughened. By increasing the borax content, small pits appear at the surface which increase in size, compare to Fig. 4b and c. The pits of the sample treated with 15 mg borax are on average (28.3 ± 16.9) nm long and increase to about (69.6 ± 44.6) nm at 30 mg borax. The pits appear slightly elongated and aligned along the carbon fiber direction, probably due to a structural anisotropy, which results in a preferential etching of the carbon fibers. After the carbon fiber electrodes were discharged and held at 1.5 V for 24 h, the surface appears much smoother and most of the small pits are filled with a reaction product. This selectively behaviour is likely to be attributed to a change of surface groups on the carbon felt after the borax treatment. These groups can serve as initial nucleus to form Li2O2 crystals. In addition deposits can be found on the tube surface. In the case of the 5 mg sample the deposits are only a few nm in size and are rather undefined in shape. In contrast, the 15 mg borax sample is covered with a layer of small plates and needles. Similar structures have been reported for discharged lithium–air cathodes by other groups and have been attributed to Li2O2.25 However, in these reports the plates and needles did overlap much more strongly making it hard to tell their exact shape and orientation. In contrast, individual plates can be observed in Fig. 4e, which stand vertically on the fiber, having only a narrow interface with the fiber surface. This is in stark contrast to the general opinion that a closed Li2O2 film is formed on the carbon surface and that the increasing film thickness is limiting charge transfer and eventually stops the electrochemical reaction. Another interesting observation is, that the morphology of the deposits is changing for the electrode treated with the highest borax content. For this sample fewer but larger mostly rhombic crystals can be observed. The crystals are about 200 nm in length and the angles of the edges are close to 60° and 120° respectively. The reported crystal structure of Li2O is cubic and of Li2O2 hexagonal. Hence, the shape of the crystals suggests that they are Li2O2. This interpretations is further supported by XPS analysis (see ESI, S-5 tables†) and an EDX mapping.
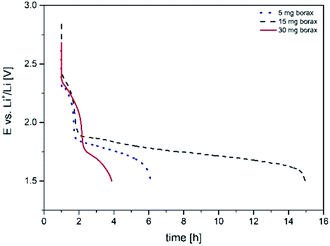 | ||
| Fig. 3 Galvanostatic discharge of carbon felt electrodes with different borax content with 50 mA g−1. | ||
XPS analysis also shows that there is no change of the boron binding energy after discharge, which means that B2O3 remains on the electrode surface and hence must be considered electrochemically inactive.
An EDX map over an area of (2.85 × 2.54) μm2 with around 50 crystals on the carbon fiber accounted for an elemental distribution of 90.4 at% carbon, 9.3 at% oxygen and 0.27% fluorine over the whole area. While carbon and fluorine are uniformly distributed, the oxygen is mainly found in the crystals (Fig. 5). Since it is not possible that the crystals consist of almost 95 at% oxygen, the element lithium (EDX detection impossible) must be present as well. Therefore, the crystals must be composed of lithium and oxygen only. Since the lithium content cannot be determined by EDX the crystals could either be Li2O or Li2O2. The lithium oxygen ratio determined by XPS (see ESI, S-5 tables†) shows a slightly higher lithium concentration than would be expected for pure Li2O2 indicating a mixture of both lithium oxygen compounds.
 | ||
| Fig. 5 EDX mapping of carbon and oxygen of the carbon fiber electrode treated with 30 mg of borax after discharge in the cell. | ||
It is not yet clear why the change in the Li2O2 growth mode occurs. Obviously the higher borax content results in a change of interface energies and as a consequence in a different shape expression of the Li2O2 crystals. This observation may help to more precisely control the way Li2O2 is deposited inside a lithium–air battery cathode which may lead to an increased capacity or capacity retention.
Conclusions
An unconventional thermal method employing borax within a closed quartz ampule under high vacuum in a pottery kiln at 800 °C for the preparation of porous carbon materials is reported. The yet unreported chemistry of borax under extreme conditions with carbon leads to a highly controllable etching of the carbon surface and may open a new fields of borax chemistry to modify carbon surfaces.The new method was used to modify a commercially available carbon felt cathode for the use in lithium–air batteries. The resulting cathodes performed more effective than the original material, which can be attributed to an increased carbon surface and maybe the creation of additional surface groups.
Depending on the borax content, two different growth modes were observed for the deposition of Li2O2 crystals, plate-like and rhombic.
Acknowledgements
C. B.-K. acknowledges continued financial support from the Karlsruhe Institute of Technology (KIT) in the context of the Helmholtz BIF and STN programs and the German Research Council (DFG). A. P. V. acknowledges financial support from the AvH Foundation. F. S. and A. F. acknowledge financial support from the KIT and DFG (SCHE 1714/1-1).Notes and references
- J. Lu, L. Li, J.-B. Park, Y.-K. Sun, F. Wu and K. Amine, Chem. Rev., 2014, 114, 5611 CrossRef CAS PubMed.
- Z. Zhang, L. Su, M. Yang, M. Hu, J. Bao, J. Wei and Z. Zhou, Chem. Commun., 2014, 50, 776 RSC.
- Z. Jian, P. Liu, F. Li, P. He, X. Guo, M. Chen and H. Zhou, Angew. Chem., Int. Ed., 2014, 53, 442 CrossRef CAS PubMed.
- D. Zhai, H.-H. Wang, J. Yang, K. C. Lau, K. Li, K. Amine and L. A. Curtiss, J. Am. Chem. Soc., 2013, 135, 15364 CrossRef CAS PubMed.
- Q. Li, P. Xu, W. Gao, S. Ma, G. Zhang, R. Cao, J. Cho, H.-L. Wang and G. Wu, Adv. Mater., 2014, 26, 1378 CrossRef CAS PubMed.
- F. Li, D.-M. Tang, Z. Jian, D. Liu, D. Golberg, A. Yamada and H. Zhou, Adv. Mater., 2014, 26, 4659 CrossRef CAS PubMed.
- D. Wu, Z. Guo, X. Yin, Q. Pang, B. Tu, L. Zhang, Y.-G. Wang and Q. Li, Adv. Mater., 2014, 26, 3258 CrossRef CAS PubMed.
- Q. Li, P. Xu, B. Zhang, H. Tsai, J. Wang, H.-L. Wang and G. Wu, Chem. Commun., 2013, 49, 10838 RSC.
- X. Han, Y. Hu, J. Yang, F. Cheng and J. Chen, Chem. Commun., 2014, 50, 1497 RSC.
- J. Shui, F. Du, C. Xue, Q. Li and L. Dai, ACS Nano, 2014, 8, 3015 CrossRef CAS PubMed.
- D. Sun, Y. Shen, W. Zhang, L. Yu, Z. Yi, W. Yin, D. Wang, Y. Huang, J. Wang, D. Wang and J. B. Goodenough, J. Am. Chem. Soc., 2014, 136, 8941 CrossRef CAS PubMed.
- H.-D. Lim, H. Song, J. Kim, H. Gwon, Y. Bae, K.-Y. Park, J. Hong, H. Kim, T. Kim, Y. H. Kim, X. Lepro, R. Ovalle-Robles, R. H. Baughman and K. Kang, Angew. Chem., Int. Ed., 2014, 126, 4007 CrossRef.
- Z.-L. Wang, D. Xu, J.-J. Xu and X.-B. Zhang, Chem. Soc. Rev., 2014, 43, 7746 RSC.
- T. S. L. Narasimhan, R. Viswanathan and S. Nalini, J. Phys. Chem. B, 2011, 115, 13261 CrossRef PubMed.
- Z. T. Zhang, S. Sridhar and J. W. Cho, ISIJ Int., 2011, 51, 80 CrossRef CAS.
- M. Binnewies, R. Glaum, M. Schmidt and P. Schmidt, Chemische Transportreaktionen, de Gruyter, 2011 Search PubMed.
- E. J. Opila, N. S. Jacobson, D. L. Myers and E. H. Copland, JOM, 2006, 58, 22 CrossRef CAS.
- N. Jacobson, S. Farmer, A. Moore and H. Sayir, J. Am. Ceram. Soc., 1999, 82, 393 CrossRef CAS.
- I. Waclawska, J. Therm. Anal., 1995, 43, 261 CrossRef CAS.
- M. Binnewies and E. Milke, Thermochemical Data of Elements and Compounds, Wiley, Weinheim, 2002 Search PubMed.
- D. J. Meschi, W. A. Chupka and J. Berkowitz, J. Chem. Phys., 1960, 33, 530 CrossRef CAS.
- M. Binnewies, Chemische Gleichgewichte, Verlag Chemie, Weinheim, 1996 Search PubMed.
- R. Köppe, P. Henke and H. Schnöckel, Angew. Chem., Int. Ed., 2008, 47, 8740 CrossRef PubMed.
- Y. C. Lu and Y. Shao-Horn, J. Phys. Chem. Lett., 2013, 4, 93 CrossRef CAS PubMed.
- R. R. Mitchell, B. M. Gallant, Y. Shao-Horn and C. V. Thompson, J. Phys. Chem. Lett., 2013, 4, 1060 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra05685g |
| ‡ Research concept was designed by Andrew P. Vogt. |
| This journal is © The Royal Society of Chemistry 2016 |