Folic acid targeted pH-responsive amphiphilic polymer nanoparticles conjugated with near infrared fluorescence probe for imaging-guided drug delivery†
Abstract
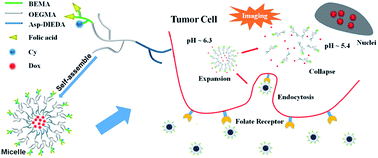
A novel folic acid targeted pH-responsive amphiphilic polymer conjugated with near infrared (NIR) probe has been synthesized by the combination of reversible addition-fragmentation chain transfer (RAFT) polymerization of 2-(N-tert-butoxycarbonylamino)ethyl methacrylate (BEMA) and oligo(ethylene glycol)methacrylate (OEGMA), ring-opening polymerization of N-carboxy anhydride (NCA) and click reaction. After assembling to micelles, the polymeric nanoparticles has pH-responsive properties for delivery encapsulated drug into the tumor cell via a rapid process of expansion and subsequent disassembly. Folic acid linked to the amino groups on the branched chains would promote the endocytosis of the polymeric nanoparticles by the cells leading the improvement of the effect of doxorubicin (DOX). In vitro experiments on both MCF7 and HepG2 cells revealed the as-prepared polymer might be a potential candidate for future theranosic against cancer.


 Please wait while we load your content...
Please wait while we load your content...