Litchi-like CdS/CdTiO3–TiO2 composite: synthesis and enhanced photocatalytic performance for crystal violet degradation and hydrogen production†
Yixuan Liab,
Wenzhi Zhang*ab,
Li Li*abc,
Chunxiong Yib,
Haiyuan Lva and
Qiang Songab
aCollege of Materials Science and Engineering, Qiqihar University, Qiqihar 161006, PR China. E-mail: qqhrll@163.com; qqhrlili@126.com; Tel: +86-0452-2738206
bCollege of Chemistry and Chemical Engineering, Qiqihar University, Qiqihar 161006, PR China
cCollege of Heilongjang Province Key Laboratory of Fine Chemicals, Qiqihar University, Qiqihar 161006, PR China
First published on 13th May 2016
Abstract
Using the programmed temperature hydrothermal or microwave-assisted hydrothermal method combined with the ion exchange method, a series of CdS/CdTiO3–TiO2 composites were prepared by adjusting the Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti element molar ratio and microwave synthesis conditions. The crystal structure, morphology, and surface physicochemical properties of the as-prepared composites were well characterized by X-ray diffraction (XRD), UV-visible diffuse reflectance spectroscopy (UV-vis/DRS), X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM), high-resolution transmission electron microscopy (HRTEM), and N2 adsorption–desorption measurements. The results showed that the composites prepared under different synthesis conditions contained mixed crystal phases of CdS, CdTiO3 and TiO2; moreover, compared to samples prepared by the programmed temperature hydrothermal method, the conditions of the microwave-assisted hydrothermal synthesis showed greater impact on the as-prepared CdTiO3 phase. The as-prepared composites basically showed a litchi-like structure, in which CdS/CdTiO3–TiO2 synthesized by the programmed temperature hydrothermal method had a smoother surface, but the dispersibility of the particles was not favorable, while compared with the composites just mentioned above, the CdS/CdTiO3–TiO2 composites prepared by the microwave-assisted hydrothermal method possessed a coarser surface, more irregular particles and better separation conditions. Simultaneously, compared with the monomer CdS, the Brunauer–Emmett–Teller surface area of these composites increased remarkably, and the optical absorption extended to the visible region. Moreover, a series of CdS/CdTiO3–TiO2 composites were tested under multiple conditions including ultraviolet light, visible light, simulated solar light and microwave-assisted irradiation to study the photocatalytic properties towards crystal violet (CV) photodegradation. The results showed that the composites presented the best photocatalytic activity when the microwave synthesis conditions were 160 °C for 6 h with 0.4
Ti element molar ratio and microwave synthesis conditions. The crystal structure, morphology, and surface physicochemical properties of the as-prepared composites were well characterized by X-ray diffraction (XRD), UV-visible diffuse reflectance spectroscopy (UV-vis/DRS), X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM), high-resolution transmission electron microscopy (HRTEM), and N2 adsorption–desorption measurements. The results showed that the composites prepared under different synthesis conditions contained mixed crystal phases of CdS, CdTiO3 and TiO2; moreover, compared to samples prepared by the programmed temperature hydrothermal method, the conditions of the microwave-assisted hydrothermal synthesis showed greater impact on the as-prepared CdTiO3 phase. The as-prepared composites basically showed a litchi-like structure, in which CdS/CdTiO3–TiO2 synthesized by the programmed temperature hydrothermal method had a smoother surface, but the dispersibility of the particles was not favorable, while compared with the composites just mentioned above, the CdS/CdTiO3–TiO2 composites prepared by the microwave-assisted hydrothermal method possessed a coarser surface, more irregular particles and better separation conditions. Simultaneously, compared with the monomer CdS, the Brunauer–Emmett–Teller surface area of these composites increased remarkably, and the optical absorption extended to the visible region. Moreover, a series of CdS/CdTiO3–TiO2 composites were tested under multiple conditions including ultraviolet light, visible light, simulated solar light and microwave-assisted irradiation to study the photocatalytic properties towards crystal violet (CV) photodegradation. The results showed that the composites presented the best photocatalytic activity when the microwave synthesis conditions were 160 °C for 6 h with 0.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (40%) Cd
1 (40%) Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti molar ratio. Meanwhile, the composite also had an excellent photocatalytic hydrogen production capacity. According to trapping experimental results, we proposed the possible mechanism of the photodegradation and photocatalytic hydrogen production in aqueous systems.
Ti molar ratio. Meanwhile, the composite also had an excellent photocatalytic hydrogen production capacity. According to trapping experimental results, we proposed the possible mechanism of the photodegradation and photocatalytic hydrogen production in aqueous systems.
In recent years, the human environment have been increasingly seriously damaged by more and more environmental problems. The traditional methods of water treatment, such as carbon adsorption, coagulation, and activated sludge have some deficiencies in the actual treatment process, making it difficult to decompose pollutants completely, and sometimes also causing secondary pollution. Therefore, there is an urgent need to find an economical and effective way to completely degrade water contaminants. Photocatalytic oxidation technology, due to its green and environment-friendly features, is becoming a promising method for pollutant treatment. Since the 1970s, researchers have found that thousands of refractory organic compounds can be degraded under sunlight or artificial light, via TiO2, ZnO or other photocatalysts.1 As a common photocatalytic material, TiO2 can absorb ultraviolet radiation to arouse electronic transitions, and then the formed photogenerated electrons and holes can react with dissolved oxygen adsorbed on the surface of the catalyst and water molecules to form strongly oxidizing OH˙ and ˙O2−; thus, high photocatalytic activity can be obtained, thereby mineralizing organic contaminant compounds.
TiO2 is cheap and easily obtained, but it has a wide band gap (3.2 eV); thus, it can be excited only under ultraviolet wavelengths less than 380 nm, which will effect the practical application of TiO2 to a degree. Recently, in order to improve the light absorption property of TiO2, some researchers have begun to modify TiO2 by recombination and doping methods. Hamed Eskandarloo et al. combined CeO2 and TiO2 to obtain optimal performance from CeO2/TiO2 composites.2 Jiang synthesized CdS -modified mesoporous TiO2 core/shell microspheres (CdS/CS-TiO2) with a strong visible light activity by an in situ synthesis method, and the sample showed excellent photocatalytic properties for the degradation of RhB and 4-CP under visible light irradiation.3
CdS is a type of IV semiconductor with a band gap of 2.4 eV at room temperature. CdS has good photoelectric properties when the wavelength is more than 463 nm. Taking cadmium acetate as the cadmium source and H2S as the sulfur source, D. W. Jing et al. obtained a CdS photocatalyst with high photocatalytic activity towards hydrogen evolution from water splitting.4 However, as one kind of photocatalyst, CdS also has the problem of lower quantum efficiency and light corrosion in practical applications. Therefore, combining CdS and TiO2 can not only expand the spectral response range of the composite, but also improve the stability of the material. Dipankar Barpuzary et al. successfully synthesized a one-dimensional CdS@TiO2 core–shell nanocomposite material by two-step hydrothermal methods,5 and the lifetime of the carrier could be prolonged when the sample was irradiated with visible light, and the photocatalytic activity was improved.
In addition, compared with the traditional hydrothermal synthesis method, the microwave-assisted hydrothermal method has some characteristics including fast heating speed, uniform heating and high utilization rate of the heat energy. In recent years, microwave heating has been widely used in chemical reactions and the synthesis of materials, because microwave synthesis can optimize the product morphology and improve a variety of material properties.
Therefore, in this paper, a series of CdS/CdTiO3–TiO2 composite materials were fabricated by the programmed temperature hydrothermal method or microwave-assisted hydrothermal combined with the ion exchange method, and the main research aims were: (1) modify the optical absorption properties of TiO2, by combining CdS and CdTiO3 to enhance the optical absorption of the TiO2-based composites, then further improve the photocatalytic properties of the composites and reduce the photocorrosion of CdS. (2) Explore the effect of Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti ratio on the photocatalytic performance. (3) Investigate the changing of experimental conditions to optimize the performance of the as-prepared composites. According to the comparison of the samples synthesized by the programmed temperature hydrothermal method and microwave-assisted hydrothermal method, by changing the synthesis temperature and microwave irradiation time, some effects of the microwave properties on the samples were researched. We expect that these effects of the microwave irradiation time and microwave temperature on the crystal size, band gap energy, specific surface area and surface morphology of samples can be revealed.
Ti ratio on the photocatalytic performance. (3) Investigate the changing of experimental conditions to optimize the performance of the as-prepared composites. According to the comparison of the samples synthesized by the programmed temperature hydrothermal method and microwave-assisted hydrothermal method, by changing the synthesis temperature and microwave irradiation time, some effects of the microwave properties on the samples were researched. We expect that these effects of the microwave irradiation time and microwave temperature on the crystal size, band gap energy, specific surface area and surface morphology of samples can be revealed.
1. Experimental
1.1. Materials and instruments
Titanium(IV) isopropoxide (TTIP, 98%) was purchased from New Jersey Co. Ltd., USA. Cadmium chloride and thioacetamide (TAA) were purchased from Kaitong Chemical Reagent Co. Ltd., Tianjin, China. Analytical grade crystal violet (CV), methyl orange (MO), rhodamine B (RhB), and acid fuchsin (AF) were used as received without further purification. Double-distilled water was used in the experiments. The MDS-8G microwave reactor was purchased from Xin Yi Co., Shanghai, China.1.2. Preparation of CdS/CdTiO3–TiO2 composite
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti in the two synthesis methods, the samples synthesised by the programmed temperature hydrothermal method were marked as X% HCT 160-6, and the samples synthesised by the microwave-assisted hydrothermal method were marked as X% WCT (X% indicates the molar ratio of Cd
Ti in the two synthesis methods, the samples synthesised by the programmed temperature hydrothermal method were marked as X% HCT 160-6, and the samples synthesised by the microwave-assisted hydrothermal method were marked as X% WCT (X% indicates the molar ratio of Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti, for example, 0.4
Ti, for example, 0.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 = 40%); a series of CdS/CdTiO3–TiO2 composites with different microwave times and temperatures were also prepared, marked as WCT temperature–time (for example, WCT 160-6).
1 = 40%); a series of CdS/CdTiO3–TiO2 composites with different microwave times and temperatures were also prepared, marked as WCT temperature–time (for example, WCT 160-6).1.3. Photocatalytic activity test
In order to investigate the photocatalytic activity of the composite materials, crystal violet (CV) was chosen as a model molecule. The UV photocatalytic reaction apparatus and light source apparatus with a built-in light source consist of a cylindrical quartz outer tube and quartz sleeve surrounding. The catalyst and the suspension of the reaction solution were surrounded by the light source. The reaction temperature was controlled by cooling water at 20 ± 5 °C; an Hg lamp was used as the UV light source (125 W Hg lamp with the maximum emission at 313.2 nm). The visible light was obtained from a 400 W Xe lamp with the main emission lines greater than 410 nm. A microwave-assisted photocatalytic reaction device and an H-shaped 15 W microwave discharge electrodeless lamp (MDEL) with the maximum emission at 280 nm were used. A simulated sunlight source was made by using a 1000 W Xe-lamp with emission from UV to near IR spectra (ca. 300–1050 nm), and the distance between the lamp and the reaction liquid was 8.5 cm. The reaction system was kept at a constant temperature by ethanol cycling.The concentration of the reaction solution (CV) was 50 mg L−1. The amount of catalyst used for the UV and simulated sunlight photodegradation was identical at 0.15 g, and the volume of solvent was 90 mL. The amount of photocatalyst for the microwave-assisted photodegradation was 0.5 g, and the volume of solvent was 500 mL. The photocatalyst was dispersed into the solution by ultrasonication for 10 min and stirred in the dark for 30 min to obtain the adsorption–desorption equilibrium between the photocatalyst and organic pollutant. At given time intervals, 3 mL suspension was collected and centrifuged to remove the photocatalyst particles. All samples were analyzed using a UV-visible spectrophotometer (TU-1901).
1.4. Photocatalytic hydrogen evolution test
Photocatalytic hydrogen evolution experiments were carried out in a reactor with a closed and connected vacuum circulatory system (LabSolar-IIIAG, purchased from Beijing Perfect Light, China). 100 mg photocatalyst was dispersed in 50 mL distilled water, and 12.5 mmol Na2S·9H2O and 17.5 mmol Na2SO3 were added as sacrificial agents. After vacuum degassing, the photocatalytic hydrogen evolution test started with lighting and stirring. A 300 W Xe lamp (PLS-SXE 300, purchased from Beijing Perfect Light, China), fixed at a distance of 10 cm from the reaction solution, was used as the light source; high purity nitrogen as a carrier gas was at a flow rate of 0.5 mL s−1; the output pressure was 0.4–0.5 MPa, and the working voltage and working current were about 20 mV and 50 mA, respectively. In the reaction process, the temperature of the reactor was kept at 5 °C by the circulating cooling water. Gas was collected after a certain irradiation time by using online gas chromatography (GC-7900, purchased from Shanghai Techcomp, China) to analyze the hydrogen production. The column was a 5 Å molecular sieve column, and the detector was a thermal conductivity detector (TCD). According to the peak area of the different reaction times, the production of hydrogen was calculated, and the catalytic activity of the photocatalyst was measured from the total hydrogen production over 8 h.2. Results and discussion
2.1. XRD
In order to investigate the crystal structure of the as-prepared composites and the effect of the microwave synthesis conditions on the crystal size of CdS/CdTiO3–TiO2, X-ray diffraction tests were employed, and the results are shown in Fig. 1 and 2. | ||
| Fig. 1 XRD patterns of TiO2, CdS and different CdS/CdTiO3–TiO2 composites prepared by programmed temperature hydrothermal synthesis. | ||
The XRD patterns of TiO2, CdS and X% HCT 160-6 composites synthesized by the programmed temperature hydrothermal method in different proportions are as shown in Fig. 1. The peaks at 2θ values of 24.9°, 43.8°, 47.9° and 51.9° correspond to the (100), (110), (103) and (112) planes of the CdS hexagonal phase (JCPDS41-1049).6 Simultaneously, those diffraction peaks at 2θ values of 25.31° (101) and 48.02° (200) indicate that the main phase of TiO2 is anatase (JCPDS 21-1272).7 The peaks at 2θ values of 27.5°, 36.1°, 41.3° and 54.3° correspond to the (110), (101), (111) and (211) planes of rutile TiO2 (JCPDS84-1283). As shown in Fig. 1, different proportions of CdS/CdTiO3–TiO2 have characteristic diffraction peaks of CdS and TiO2 at the same time, the peaks at 2θ values of 31.1°, 34.2° and 41.3° belonging to the CdTiO3 ilmenite phase (JCPDS 85-0452). Currently, there is only little study according to relevant reports,8,9 CdTiO3 often has higher stability, which can make up for the influence of CdS light corrosion during the reaction, and CdTiO3 is also a kind of photocatalyst, whose presence can improve the photocatalytic activity of the composite to a certain extent. What we can observe from Fig. 1 is that the characteristic peaks of CdTiO3 in the 40% HCT sample were more evident and sharper, indicating that the CdTiO3 in the HCT sample was more perfect than that in the other proportion samples prepared by the programmed temperature hydrothermal method. Moreover, the average crystallite sizes of these CdS/CdTiO3–TiO2 composites, estimated using the Scherrer equation, are listed in Table S1 (ESI†). From Table S1,† with the increase in Cd molar ratio, the crystallite sizes of the samples prepared by the temperature programmed hydrothermal method show some trends: small → big → small; among them, the crystallite size of the sample of 40% HCT 160-6 is the largest. According to the relevant literature,10 the increase in crystallite sizes can effectively improve the photocatalytic activity, and these reports are consistent with our photocatalytic test results. Accordingly, the sample ratio was fixed at 40% in follow-up experiments using the microwave-assisted hydrothermal method.
The XRD patterns of TiO2, CdS and composites synthesized by the microwave-assisted hydrothermal method under different synthesis conditions are presented in Fig. 2(a) and (b). As shown in Fig. 2, the peak at a 2θ value of ca. 25.3° (101) is a typical characteristic peak of anatase TiO2;11 at the same time, the peaks at 27.5° (110), 36.1° (101), 41.3° (111) and 54.3° (211) are some typical characteristic peaks of rutile TiO2, and the peaks at 2θ values of 36.7° and 43.8° correspond to the (102) and (110) planes of CdS (JCPDS41-1049), respectively.12,13 Compared with the as-mentioned monomers, the characteristic diffraction peaks of TiO2 and CdS are found in the composites, simultaneously. The CdS/CdTiO3–TiO2 composites prepared under different microwave-assisted synthesis conditions possess the characteristic peaks of CdTiO3, appearing at 31.1°, 41.3° and 34.2°. This indicates that composites treated with microwaves also exist in three mixed crystal phases of TiO2, CdTiO3 and CdS. By analysing Fig. 2, we can observe that with the increase in microwave radiation time, the characteristic peak of CdTiO3 gradually becomes higher and sharper, attributed to the polarization during microwave synthesis, which can lead to a greater degree of perfect crystallinity. Of course, it is also one of the reasons that the photocatalytic activity is increased. According to Fig. 2(b), the intensity of the characteristic CdTiO3 peak shows some change with the increase in temperature, indicating that the microwave temperature can influence the formation of CdTiO3 to a certain extent.
In summary, from the comparison of the two synthetic methods, it can be found that the diffraction peaks of the samples prepared by microwave hydrothermal method are higher and sharper, attributed to the microwave polarization effect on the crystal growth. The average crystallite sizes of CdS/CdTiO3–TiO2 calculated using the Scherrer formula14 are listed in Table S1 (ESI†). Compared with the monomer TiO2, with the CdS doping amount increasing, the crystallite size of the composite was changed from small to large and then to small again. The crystallite sizes of CdS/CdTiO3–TiO2 synthesized by the microwave-assisted hydrothermal method were generally larger than those of samples prepared by the hydrothermal synthetic method, indicating that the microwave hydrothermal treatment can promote the growth of the crystals. Simultaneously, we found that the crystallite size of the sample increased in varying degrees accompanied with the increase in the microwave synthesis temperature, which further showed that the microwave synthesis temperature had a certain effect on the crystallinity of the sample. According to the relevant reports,15–17 the reduction in crystallite size leads to the quantization effect, altering the electric field gradients and reducing the separation of electron–hole pairs.18 Almquist and her co-workers reported that the photocatalytic performance of the nanoparticles improved upon an increase in the crystallite size due to the optimization of the optical properties and charge carrier dynamics.10 Moreover, the calculated lattice parameters of CdS/CdTiO3–TiO2 can be seen in Table S1 (ESI†). From Table S1 (ESI†), it can be observed, compared with pure TiO2, that the lattice parameters of the composites had obviously changed with the increase in CdS doping amount, indicating that some of the Cd ions had doped into the TiO2 lattices and generated CdTiO3, resulting in some changes in the lattice parameters.
2.2. UV-visible diffuse reflectance spectroscopy
The effect of the two kinds of synthetic method and the microwave-assisted synthesis conditions (temperature and time) on the optical absorption properties of the CdS/CdTiO3–TiO2 samples were explored by UV-visible diffuse reflectance, as shown in Fig. 3 and S1 (ESI†). Fig. S1a (ESI†) shows the UV-vis/DRS results of CdS, TiO2 and different proportion of CdS/CdTiO3–TiO2 composites prepared by the programmed temperature hydrothermal method. The absorption edge of TiO2 is located only in the UV region < 320 nm, whereas pure CdS shows a strong absorbance in the visible region.19,20 However, all composite samples produce a certain absorption intensity in the visible region. According to the absorption edge of CdS and TiO2, it’s not difficult to find that the optical absorbance properties of the CdS/CdTiO3–TiO2 composites show greater difference. The optical absorption of the sample of 40% HCT 160-6 is located between 430 and 500 nm, which is significantly higher than that of the other composites, indicating that it will show higher visible light photocatalytic activity. | ||
| Fig. 3 UV-vis/DRS spectra (a) and the plot of transformed Kubelka–Munk function vs. absorption energy of light (b) of CdS/CdTiO3–TiO2 prepared by two methods. | ||
The energy band gaps of all the samples were calculated from their optical absorption edges in the UV-vis/DRS spectra using the following equation:21
| (αhν)n = K(hν − Eg) |
2.3. X-ray photoelectron spectroscopy
The surface elemental composition and the chemical state of the CdS/CdTiO3–TiO2 composite have been analyzed by XPS, and the obtained results are shown in Fig. 4. The typical spectrum of the sample clearly shows the presence of O, Ti, Cd, S, and C elements, where the observed carbon peak was due to absorbed gas molecules as an impurity.23 | ||
| Fig. 4 XPS full spectrum of the 40% WCT 160-6 composite (a) and patterns of O1s (b), S2p (c), Ti2p (d) and Cd3d (e). | ||
The O1s peak observed at a binding energy of 530.7 eV is attributed to O1s originating from bulk lattice oxygen, as shown in Fig. 4(b).24,25 Characteristic peaks of S2p3/2 (161.7 eV) and S2p1/2 (162.5 eV) confirm the presence of S in the −2 valence state, as shown in Fig. 4(c). The XPS spectrum of Ti is shown in Fig. 4(d), and the binding energy positions located at 459.5 and 465.3 eV are ascribed to Ti2p3/2 and Ti2p1/2 in the CdS/CdTiO3–TiO2 (40% WCT 160-6) sample, which are consistent with the binding energies of Ti4+.26 Fig. 4(e) shows that Cd3d mainly consists of 3d5/2 and 3d3/2 spin–orbit components at 405.8 and 412.5 eV, respectively, indicating the presence of Cd in the +2 valence state.27–29 According to the relative peak intensities of Cd3d, Ti2p, O1s and S2p in the spectra, the relative contents of Cd, Ti, O and S are about 8.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62
62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.9, which were calculated by the sensitivity factor method.30 The relative content ratio of Ti
8.9, which were calculated by the sensitivity factor method.30 The relative content ratio of Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O = 10
O = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 can prove that the photocatalyst contains TiO2 and TiO32−, which is consistent with the XRD results.
31 can prove that the photocatalyst contains TiO2 and TiO32−, which is consistent with the XRD results.
2.4. Morphology
In order to investigate the surface morphologies of the composite materials, 5% HCT 160-6, 40% HCT 160-6, 40% WCT 160-6, WCT 100-6 and WCT 160-1 were analyzed by scanning electron microscopy.Fig. 5 shows the SEM images of the 5% HCT 160-6, 40% HCT 160-6 and 40% WCT 160-6 composite materials at different typical scales. Fig. 5(a)–(f) show that the surface morphologies of the CdS/CdTiO3–TiO2 composites prepared by the temperature programmed hydrothermal treatment consist of spherical, irregular nanoparticles accumulating on the sphere outer surface, and both the uniformity of the sphere size and the surface smoothness decrease with the increase in CdS doping amount. Fig. 5(e) shows that most of the 40% HCT 160-6 spheres coincide with each other, without favorable separation. Fig. 5(g)–(i) show that the composites present a lichi-like structure with a relatively uniform size and rough surface, and the irregular particle accumulation can be observed on the relatively independent sphere outer surfaces; all these features are conducive to increasing the contact area between catalysts and reactants, which is then expected to improve the photocatalytic activities of the composites. Fig. 5(j)–(l) show SEM images of 40% WCT 100-6 at different typical scales. Compared with 40% WCT 160-6, although 40% WCT 100-6 exhibits a lichi-like structure, the sphere is irregular and doesn’t have good separation. Fig. 5(m)–(o) show SEM images of 40% WCT 160-1 at different typical scales. Compared with 40% WCT 160-6, the synthesized 40% WCT 160-1 consists of irregular spheres with poor separation and more irregular particles existing among the spheres under these synthetic conditions, which is probably due to the microwave radiation time being too short to accumulate particles to form spheres.
 | ||
| Fig. 5 SEM images of composites (a–c) 5% HCT; (d–f) 40% HCT; (g–i) 40% WCT 160-6; (j–l) 40% WCT 100-6 and (m–o) 40% WCT 160-1. | ||
The morphological characteristics and the crystal structures of CdS/CdTiO3–TiO2 (40% WCT 160-6) are further investigated by HR-TEM and the results are illustrated as Fig. 6. Fig. 6(a)–(c) are the images of the edge and middle part of the spheres. Through use of software (Gatan Digital Micro group), we estimate the fringe spacing (as is shown in Fig. 6(d)–(j)). Several sets of fringe spacings are ca. 0.287, 0.385, 0.324, 0.352, 0.334, 0.207 and 0.176 nm, corresponding to CdTiO3 (211), CdTiO3 (110), rutile TiO2 (110), anatase TiO2 (101), CdS (002), CdS (110) and CdS (112), respectively. As is shown in Fig. 6(d), a hexagon can be observed from the FFT image, according to the FFT line plot. We find an obvious hexagonal CdS lattice fringe, which is taken as proof that CdS is indeed in the hexagonal phase, consistent with the XRD results. From the enlarged drawing of Fig. 6(c) (directional arrow in Fig. 6(c)), there are obvious dividing lines among the two sets of lattice fringes, which thus proves the as-prepared composites have rutile TiO2 and anatase TiO2 heterojunctions.
 | ||
| Fig. 6 HR-TEM images of the edge and middle part of a sphere (a–c), FFT line plot and FFT (d–j) of 40% WCT sample. | ||
2.5. N2 adsorption–desorption isotherms
In order to investigate the physicochemical properties of the different samples, the adsorption–desorption isotherm type, pore size distribution, surface area, total pore volume and average pore diameter of CdS/CdTiO3–TiO2, CdS, TiO2 and 40% WCT with different microwave synthesis conditions were investigated by N2 adsorption–desorption measurements, and the related results are shown in Fig. 7 and Table S3 (ESI†). As shown in Fig. 7, the N2 adsorption–desorption isotherms of CdS, TiO2, 40% HCT 160-6 and 40% WCT 160-6 reveal a type IV isotherm with H3 hysteresis loop according to the IUPAC classification, indicating the presence of mesopores.24 The type of isotherm and the hysteresis loop are caused by the condensation of the capillary and the aggregation of particles. | ||
| Fig. 7 Nitrogen adsorption–desorption isotherms and Barrett–Joyner–Halenda (BJH) pore size distributions (insets) of different samples. | ||
According to Table S3 (ESI†), the BET specific surface area of 40% WCT 160-6 is less than that of 40% 160-6 HCT, which is probably due to the 40% WCT 160-6 spheres being relatively independent, while the 40% HCT 160-6 spheres are not completely separated from each other. These results are also consistent with the SEM images. In Fig. 7, the illustrations of the BJH pore size distribution curves can be seen. Compared with the pore size distribution of 40% 160-6 WCT, that of 40% 160-6 HCT is more uniform, which should be attributed to the programmed temperature hydrothermal method being relatively mild in the synthesis process, leading to the internal structure of the photocatalyst being more uniform. However, the microwave-assisted hydrothermal synthesis reaction is very vigorous and quickly forms the catalyst, which could cause the non-uniform pore size distribution. The BET specific surface area, pore size distribution and total pore volume of the CdS/CdTiO3–TiO2 composites are larger than those of CdS, meaning that the composite materials based on the CdS structure have been optimized.
2.6. Photocatalytic activity of CdS/CdTiO3–TiO2
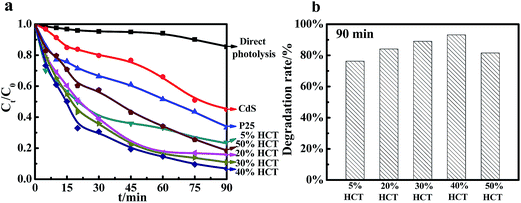
In order to investigate the photocatalytic activities of the different proportion CdS/CdTiO3–TiO2 composites prepared by programmed temperature hydrothermal synthesis, a series of experiments were carried out, including direct photolysis (without photocatalyst), CdS, P25 and CdS/CdTiO3–TiO2 composites (X% HCT) degrading crystal violet under UV light; the results of the relevant photocatalytic experiments are shown in Fig. 8. As can be seen from Fig. 8, the degradation efficiencies of the different photocatalysts were diverse. The activities of X% HCT were higher than those of the monomer and direct photocatalysis, due to TiO2 having strong absorption in the ultraviolet region; after forming a composite with CdS, the synergy between TiO2 and CdS can further improve the efficiency of the photocatalytic degradation. Of course, the portion of CdTiO3 generated during synthesis also had a significant impact on catalysis. In addition, according to the experimental results of the different proportion photocatalysts, the UV photocatalytic degradation efficiency of 40% HCT was the best, and the photocatalytic activity of 50% HCT 160-6 decreased because of the reduction of the Eg value, resulting in the increase of the electron–hole pair recombination rate and the decrease of the photocatalytic quantum efficiency. Accordingly, the optimal ratio of the composite materials was 40%, which can be further confirmed.A series of profiles of composites degrading crystal violet under UV light, wherein the composites were synthesized under different microwave synthesis conditions, are shown in Fig. 9. From Fig. 9(a), it can be observed that the photocatalytic activity is best when the microwave conditions are 160 °C for 6 h. The photocatalytic activities of the optimum catalyst prepared by both the temperature programmed hydrothermal method and microwave-assisted hydrothermal method to degrade CV under UV light are shown in Fig. 9(b). It can be clearly observed that the activity of the sample synthesized by the microwave-assisted hydrothermal method is much better under the same experimental conditions. Fig. 9(c) shows that all photocatalytic degradation of CV follows second order reaction kinetics.
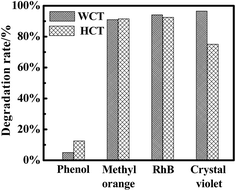
Fig. 10 is the comparison of the results of different dyes degraded under UV irradiation. According to the comparison, 40% CdS/CdTiO3–TiO2 composites prepared by the two methods have a certain universality for the degradation of different types of dyes under UV irradiation. As shown in Fig. 11, under visible light, simulated sunlight and microwave irradiation, the degradation effects of CV based on direct photocatalysis, CdS, P25, 40% HCT 160-6 and 40% WCT 160-6 are enhanced in sequence. In addition, it can be observed from Fig. S2 (ESI†) that, after several cycles of experiments, the degradation rates decreased to a certain degree. That is owing to the catalyst CdS/CdTiO3–TiO2 being a kind of sulfide-containing compound, and it is easily broken down under the high temperature treatment, leading to the decomposition of sulfide, which then results in the destruction of its composition and structure. Therefore, a purge of water and ethanol around CdS/CdTiO3–TiO2 in the recycling experiment was carried out, which meant that some dye molecules present in the pores of CdS/CdTiO3–TiO2 could not be removed completely. Because some of the pores of CdS/CdTiO3–TiO2 were blocked, this will affect its activity. Fig. S2c (ESI†) is the XRD patterns of the recycled product, and it can be observed that the composition of catalyst have no obvious changes, illustrating the catalyst can maintain the structure stability.
 | ||
| Fig. 11 Degradation of CV by different photocatalysts under visible light, simulated sunlight and microwave irradiation conditions. | ||
In order to investigate the photocatalytic hydrogen evolution ability of the as-prepared 40% WCT 160-6 photocatalyst, a series of experiments were carried out, as shown in Fig. 12. From Fig. 12, it can be clearly observed that the photocatalytic hydrogen evolution ability of TiO2 is very weak before CdS and CdTiO3 were combined with it, and this illustrates that the presence of CdS and CdTiO3 can make up for the shortcoming of TiO2 (ECB = −0.29 eV vs. NHE) that the conduction band is too close to the potential of H2/H2O (0.0 eV vs. NHE), and owing to CdS (ECB = −0.52 eV vs. NHE) being more negative, the presence of CdS and CdTiO3 in the as-prepared composite can improve its ability for hydrogen production.33
According to above activity tests, we find that the sample of 40% WCT 160-6 has the best activity, which can be attributed to: (1) the sample 40% WCT 160-6 has the best optical absorption properties according to the UV-vis/DRS results and can be excited to generate electrons and holes under the simulated sunlight irradiation; therefore, the photocatalytic reaction can occur; (2) in the microwave reactor vessel, due to the role of the high temperature, pressure and microwaves, they can make the aggregation of the catalyst particles decrease, and the particle dispersion is more uniform, thereby improving the photocatalytic activity; (3) the band gap of TiO2 is 3.2 eV, leading to its lower activity; after combining it with CdS and CdTiO3, the band gap of the composite is dramatically narrowed, and thus the activity is greatly improved.
2.7. The formation mechanism of the CdS/CdTiO3–TiO2 composite
According to the relevant experimental data, the possible growth mechanism of the CdS/CdTiO3–TiO2 composite was discussed, with the specific content shown in Scheme S1 (ESI†). CdCl2 and TTIP were mixed into a reaction kettle, in which the TiO2 nanoparticles were hydrolyzed by water to afford Ti(IV) species. The surface energy was raised along with the rising temperature during the synthesis process, leading to these particles aggregating to rapidly form the precursor. During the growth of the CdS/CdTiO3–TiO2 composite, TAA functioned as a sulfur source and a bidentate ligand to form a relatively stable Cd–TAA complex. The S–C bond of thiourea can be cleaved during the chemical bath process. The resulting sulfur ions exchanged oxygen ions to react with Cd ions until the CdS was generated.2.8. Photocatalytic mechanism
In order to explore the reaction mechanism of the as-prepared catalyst, trapping experiments with 40% WCT 160-6 were carried out, wherein the trapping agents were isopropanol and benzoquinone.31,32 As shown in Fig. S3 (ESI†), benzoquinone and isopropanol successfully captured active groups in the UV photocatalytic experiments, that is, the superoxide radical (˙O2−) and the hydroxyl radical (˙OH) played a major role during the process of photocatalytic degradation of the dyes.25,33Based on the above trapping experiments, a probable reaction mechanism was proposed (Scheme 1).
Scheme 1 is the possible photocatalytic reaction mechanism of the CdS/CdTiO3–TiO2 composite. According to the formula ECB = χ − 4.5 − 0.5Eg, the conduction band and valence band locations of CdS, CdTiO3 and TiO2 can be calculated,34 which are listed in Table 1. Under the light irradiation, photoelectrons can be excited from the valence band (VB) of CdS in CdS/CdTiO3–TiO2 to the conduction band (CB) of CdS, thus forming electron–hole pairs. Due to the formation of CdTiO3 in the synthesis process, charge transfer paths are increased, which can promote charge transfer and the separation of photogenerated electron–holes, thereby increasing the overall photoelectric conversion efficiency and photocatalytic activity. Simultaneously, because of the interfacial contact between TiO2 and CdS, photogenerated electrons can transfer to TiO2, thus enhancing the lifetimes of the charge carriers. However, accumulated holes on the VB of CdS cannot produce ˙OH radicals by the oxidization of surface hydroxyl groups or adsorbed water molecules. Similarly, the electron on the CB of TiO2 cannot reduce oxygen molecules (O2) into superoxide radicals (O2˙−). This is because the CB potential of TiO2 (−0.29 eV vs. NHE) is more positive than the redox potential of O2˙− formation (O2/O2˙− = −0.33 eV vs. NHE) and the VB potential of the CdS (+1.88 eV vs. NHE) is more negative than the potential required to oxidize H2O or –OH to ˙OH radicals (+2.4 eV vs. NHE).35,36 Therefore, in the reaction system, the molecular oxygen can be activated to form superoxide radicals on the CB of CdS, and the holes that are generated from the VB will react with H2O to form OH˙ radicals on the VB of TiO2. Thus, these reactive species can efficiently inhibit recombination of electron–hole pairs and prolong their lifetimes. The generation of OH˙ and O2˙− can be proved by trapping experiments, and they degrade and even mineralize organic pollutants to CO2 and H2O. At the same time, the conduction band position of the CdS/CdTiO3–TiO2 is more negative than the hydrogen electrode reaction potential (0.0 eV vs. NHE), which can satisfy the requirements of the hydrogen evolution reaction so that conduction band electrons may reduce water into hydrogen. Since the as-synthesized composite CdS/CdTiO3–TiO2 can simultaneously satisfy the above conditions of degradation and hydrogen production, it may have activities of both photocatalytic degradation of dye and photocatalytic hydrogen evolution.
| Potential (eV) | CdS | TiO2 | CdTiO3 |
|---|---|---|---|
| ECB | −0.52 | −0.29 | −0.68 |
| EVB | 1.88 | 2.91 | 3.22 |
3. Conclusions
A series of CdS/CdTiO3–TiO2 composites were prepared by programmed temperature hydrothermal and microwave-assisted hydrothermal methods. The results showed that the CdS/CdTiO3–TiO2 composites existed in three mixed crystal phases of TiO2, CdTiO3 and CdS. With the increase in the Cd doping amount, the crystallite size showed this trend: small → big → small. The crystallite sizes of the composites prepared by the microwave-assisted hydrothermal method were generally larger than those of the programmed temperature hydrothermal synthesis samples, and the crystallite size of the sample also increased to a degree along with the increasing microwave synthesis temperature, which indicated that the microwave hydrothermal treatment may promote the growth of crystals and had some influence on the sample crystallinity. The surface morphology of the CdS/CdTiO3–TiO2 composite prepared by the temperature programmed hydrothermal treatment was spherical with the accumulation of irregular nanoparticles on the outer surface of the spheres. Both the uniformity of the sphere size and the surface smoothness decreased with the increase in CdS doping amount. The surface morphology of the CdS/CdTiO3–TiO2 composite prepared by the microwave-assisted hydrothermal treatment showed a lichi-like structure owing to these features, like independent spherical structure, relatively uniform size, surface roughness, and the irregular particle accumulation on the sphere outer surface. Based on the results of photocatalytic degradation of CV by the CdS/CdTiO3–TiO2 composites, the best microwave synthesis conditions of the photocatalyst with the highest ultraviolet light catalytic activity were determined as 160 °C for 6 h with the molar ratio as 0.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, that is, 40% WCT 160-6. The as-prepared photocatalyst not only had the ability to degrade organic pollutants, but also exhibited excellent photocatalytic hydrogen production capacity, showing that the synergistic effect of CdS, CdTiO3 and TiO2 can help enhance its photocatalytic activity.
1, that is, 40% WCT 160-6. The as-prepared photocatalyst not only had the ability to degrade organic pollutants, but also exhibited excellent photocatalytic hydrogen production capacity, showing that the synergistic effect of CdS, CdTiO3 and TiO2 can help enhance its photocatalytic activity.
Acknowledgements
This study is supported by the National Natural Science Foundation of China (21376126), Natural Science Foundation of Heilongjiang Province, China (B201106), Scientific Research of Heilongjiang Province Education Department (12511592), Government of Heilongjiang Province Postdoctoral Grants, China (LBH-Z11108), Open Project of Green Chemical Technology Key Laboratory of Heilongjiang Province College, China (year 2013), Postdoctoral Researchers in Heilongjiang Province of China Research Initiation Grant Project (LBH-Q13172), Innovation Project of Qiqihar University Graduate Education (YJSCX2014-009X), and Qiqihar University in 2016 College Students Academic Innovation Team Funded Projects.References
- J. H. Carey, J. Lawrence and H. M. Tosine, Bull. Environ. Contam. Toxicol., 1976, 16, 697–701 CrossRef CAS PubMed.
- H. Eskandarloo, A. Badiei and M. A. Behnajady, Ind. Eng. Chem. Res., 2014, 53, 7847–7855 CrossRef CAS.
- B. Jiang, X. Yang, X. Li, D. Q. Zhang, J. Zhu and G. S. Li, J. Sol-Gel Sci. Technol., 2013, 3, 504–511 CrossRef.
- D. W. Jing and L. J. Guo, J. Phys. Chem. B, 2006, 110, 11139–11145 CrossRef CAS PubMed.
- D. Barpuzary, Z. Khan, N. Vinothkumar, M. De and M. Qureshi, J. Phys. Chem. C, 2012, 116, 150–156 CAS.
- M. Matsumura, S. Furukawa, Y. Saho and H. Tsubomura, J. Phys. Chem., 1985, 89, 1327–1329 CrossRef CAS.
- L. Chen, B. Yao, Y. Cao and K. Fan, J. Phys. Chem. C, 2007, 111, 11849–11853 CAS.
- Q. U. Yang, W. Zhou, Z. Y. Ren, K. Pan, L. Jiang and H. G. Fu, Chem. J. Chin. Univ., 2014, 5, 995–999 Search PubMed.
- X. C. Jiang, Y. L. Wang, T. Herricks and Y. N. Xia, J. Mater. Chem., 2004, 14, 695–703 RSC.
- C. B. Almquist and P. Biswas, J. Catal., 2002, 212, 145–156 CrossRef CAS.
- H. Eskandarloo, A. Badiei and M. A. Behnajady, Ind. Eng. Chem. Res., 2014, 53, 7847–7855 CrossRef CAS.
- Z. Chen and Y. J. Xu, ACS Appl. Mater. Interfaces, 2013, 5, 13353–13363 CAS.
- S. Xiong, B. Xi and Y. Qian, J. Phys. Chem. C, 2010, 114, 14029–14035 CAS.
- L. Li, L. L. Wang, W. Z. Zhang, X. L. Zhang, X. Chen and X. Dong, J. Nanopart. Res., 2014, 16, 2753 CrossRef.
- K. Y. Jung, S. B. Park and S. K. Ihm, Appl. Catal., A, 2002, 224, 229–237 CrossRef CAS.
- A. P. Rivera, K. Tanaka and T. Hisanaga, Appl. Catal., B, 1993, 3, 37–44 CrossRef CAS.
- M. Inagaki, Y. Nakazawa, M. Hirano, Y. Kobayashi and M. Toyoda, Int. J. Inorg. Mater., 2001, 3, 809–811 CrossRef CAS.
- C. Colbeau-Justin, M. Kunst and D. Huguenin, J. Mater. Sci., 2003, 38, 2429–2437 CrossRef CAS.
- L. Li, X. L. Zhang, W. Z. Zhang, L. L. Wang, X. Chen and Y. Gao, Colloids Surf., A, 2014, 457, 134–141 CrossRef CAS.
- Y. Chen, G. Tian, K. Pan, C. Tian, J. Zhou, W. Zhou, Z. Ren and H. Fu, Dalton Trans., 2012, 41, 1020–1026 RSC.
- T. I. Draskovic, M. Z. Yu and Y. Y. Wu, Inorg. Chem., 2015, 54, 5519–5526 CrossRef CAS PubMed.
- S. A. Mayén-Hernández, G. Torres-Delgado, R. Castanedo-Péreza, A. Cruz-Oreab, J. G. Mendoza-Alvarezb and O. Zelaya-Angelb, Sol. Energy Mater. Sol. Cells, 2006, 90, 2280–2288 CrossRef.
- D. Q. Zhang, M. C. Wen, B. Jiang, J. Zhu, Y. N. Huo and G. S. Li, J. Sol-GelSci. Technol., 2012, 62, 140–148 CrossRef CAS.
- L. Li, X. D. Huang, T. Y. Hu, J. X. Wang, W. Z. Zhang and J. Q. Zhang, New J. Chem., 2014, 38, 5293–5302 RSC.
- B. Erdem, R. A. Hunsicker, G. W. Simmons, E. D. Sudol, V. L. Dimonie and M. S. El-Aasser, Langmuir, 2001, 17, 2664–2669 CrossRef CAS.
- H. H. Ou, S. L. Lo and C. H. Liao, J. Phys. Chem. C, 2011, 115, 4000–4007 CAS.
- Z. Lian, P. Xu, W. Wang, D. Zhang, S. Xiao, X. Li and G. Li, ACS Appl. Mater. Interfaces, 2015, 7, 4533–4540 CAS.
- Y. Wang, Z. Tang, X. Liang, L. M. Liz-Marzán and N. A. Kotov, Nano Lett., 2004, 4, 225–231 CrossRef CAS.
- T. Nakanishi, B. Ohtani and K. Uosaki, J. Phys. Chem. B, 1998, 102, 1571–1577 CrossRef CAS.
- C. D. Wagner, J. F. Moulder, L. E. Davis and W. M. Riggs, Handbook of X-ray photoelectron spectroscopy, 1979 Search PubMed.
- Y. Chen, G. Tian, Y. Shi, Y. Xiao and H. Fu, Appl. Catal., B, 2015, 164, 40–47 CrossRef CAS.
- Y. Chen, G. Tian, T. Feng, W. Zhou, Z. Ren, T. Han, Y. Xiao and H. Fu, CrystEngComm, 2015, 17, 6120–6126 RSC.
- S. Pasternak and Y. Paz, ChemPhysChem, 2013, 14, 2059–2070 CrossRef CAS PubMed.
- Y. Xu and M. A. A. Schoonen, Am. Mineral., 2000, 85, 543–556 CrossRef CAS.
- W. K. Jo and T. S. Natarajan, ACS Appl. Mater. Interfaces, 2015, 7, 17138–17154 CAS.
- Y. Chen, G. Tian, Q. Guo, R. Li, T. Hana and H. Fu, CrystEngComm, 2015, 17, 8720–8727 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra05631h |
| This journal is © The Royal Society of Chemistry 2016 |