Synthesis and photocatalytic activity of g-C3N4/BiOI/BiOBr ternary composites
Abstract
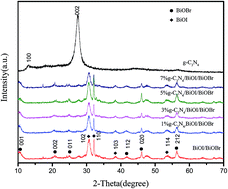
A novel ternary composite photocatalyst (g-C3N4/BiOI/BiOBr) was prepared via a facile solvothermal method. The samples were characterized by powder X-ray diffraction, transmission electron microscopy, UV-visible diffuse reflection spectrometry, X-ray photoelectron spectrometry and photoluminescence measurements. Under irradiation with visible light, the g-C3N4/BiOI/BiOBr photocatalyst showed a higher photocatalytic activity than pure g-C3N4 and BiOI/BiOBr for the degradation of methylene blue. Among the hybrid photocatalysts, 3% g-C3N4/BiOI/BiOBr showed the highest photocatalytic activity for the degradation of MB. These results suggest that the heterostructure combination of g-C3N4, BiOI and BiOBr provides a synergistic effect through an efficient charge transfer process.

 Please wait while we load your content...
Please wait while we load your content...