Mango stone-derived activated carbon with high sulfur loading as a cathode material for lithium–sulfur batteries†
Abstract
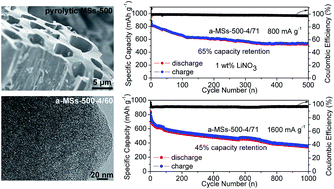
A set of mango stone (MS)-derived activated carbons with tunable pore structure parameters have been synthesized via a KOH activation procedure. The maximum specific surface area of 3334 m2 g−1 and pore volume of 2.17 cm3 g−1 are obtained for the material which is pre-carbonized at 500 °C and treated with a KOH/char mass ratio of 4 (a-MSs-500-4). Given the large pore volume of the resultant a-MSs-500-4 activated carbon, a high sulfur loading of up to 71 wt% can be achieved (a-MSs-500-4/71). When applied in a lithium–sulfur battery, the a-MSs-500-4/71 composite cathode exhibits 64% capacity retention over 500 cycles at 800 mA g−1 and 45% capacity retention over 1000 cycles at 1600 mA g−1, indicating excellent long-term cycling performance. Moreover, after 1 wt% LiNO3 is introduced as the electrolyte additive, the average coulombic efficiency of the electrode is as high as approximately 99%.

 Please wait while we load your content...
Please wait while we load your content...