A diarylethene-based fluorescent chemosensor for the sequential recognition of Fe3+ and cysteine†
Abstract
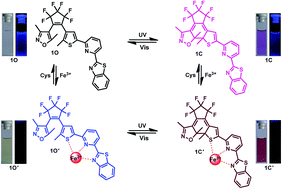
A new fluorescent chemosensor for the sequential recognition of Fe3+ and cysteine has been constructed based on photochromic diarylethene. It exhibits sequential recognition of Fe3+ and cysteine via an “on–off–on” molecular switch. The title compound can selectively and sensitively recognize Fe3+ in methanol, causing quenching of the fluorescence. Then, the formed complex was found to be a fluorescent chemosensor for cysteine with an increase in the fluorescence intensity. A detection limit as low as 4.09 × 10−8 mol L−1 for Fe3+ and 2.89 × 10−8 mol L−1 for cysteine was obtained. Moreover, a two input INHIBIT logic gate was fabricated by using Fe3+ and cysteine as inputs and taking I439 as the output.

 Please wait while we load your content...
Please wait while we load your content...