Assessment of gas production from natural gas hydrate using depressurization, thermal stimulation and combined methods
Abstract
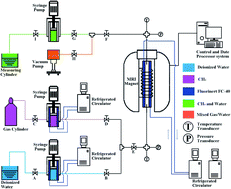
The largest sources of hydrocarbons worldwide are distributed in the permafrost and submarine sediments in the form of methane hydrates, but exploitation of these hydrocarbons is still years away from being economical, safe, and commercially viable; thus, further research is needed. To analyze the characteristics of methane hydrate (MH) dissociation and evaluate the gas production during the application of different MH decomposition methods, this study firstly compared MH dissociation during depressurization, thermal stimulation, and combined method (depressurization + thermal stimulation) treatments using magnetic resonance imaging (MRI) in situ observation. In particular, the influences of back-pressure and temperature on hydrate dissociation, the hydrate saturation, the rate of hydrate dissociation and MRI images from each of the three methods were investigated. The results proved that during application of the depressurization and combined methods at different back-pressures (2.2–2.6 MPa), the MH dissociation proceeded via radial dissociation rather than axial dissociation; moreover, during the application of the thermal stimulation method at different dissociation temperatures (278.15–288.15 K), the MH dissociated uniformly. Overall, a combination of depressurization and thermal stimulation at the initial stage of hydrate decomposition was proposed and comparison of the three methods demonstrated that the combined method had obvious advantages for methane treatment. Specifically, the combined method was capable of solving the problems related to low gas production and poor energy efficiency that were encountered when using either the depressurization or thermal stimulation method alone.

 Please wait while we load your content...
Please wait while we load your content...