Ultrathin N-rich boron nitride nanosheets supported iron catalyst for Fischer–Tropsch synthesis†
Abstract
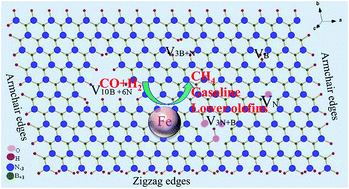
The boron nitride nanosheets (BNNSs) have attracted great interest in the field of energy storage and heterogeneous catalysis. In this paper, BNNSs supported iron (Fe/BNNSs) catalysts were prepared by one-pot solid state reaction and used in Fischer–Tropsch synthesis (FTS) for the first time. The microscopic structure, morphology and metal–support interaction of the Fe/BNNSs catalysts were investigated by TEM, FT-IR, 1H MAS NMR and H2-TPR. The average thickness of the N-rich BNNSs support was 4–8 nm, and the mean size of the iron nanoparticles was 25–40 nm. The CO conversion, CH4 and C5+ selectivity of typical Fe/BNNSs catalyst with 33 wt% Fe-loading were 47%, 13.7% and 48% at 270 °C, respectively. No obvious deactivation was observed even after 270 h running. The conversion, selectivity and the iron time yield (FTY) of Fe/BNNSs catalysts were highly related to the loading, dispersion of iron nanoparticles. The lower loading and better dispersion of the iron nanoparticles in Fe/BNNSs catalyst resulted in the better FTY and C5+ selectivity. The N-rich defects of BNNSs and porous structure of BNNSs anchored active phases to prevent them from growing larger. Therefore, the BNNSs support plays an important role in retarding the catalyst from deactivation.

 Please wait while we load your content...
Please wait while we load your content...