The crystallization behaviors and mechanical properties of poly(L-lactic acid)/magnesium oxide nanoparticle composites†
Junping Jiaa,
Jinjun Yang*ab,
Yun Zhaoa,
Hui Lianga and
Minfang Chen*a
aSchool of Materials Science and Engineering, Tianjin University of Technology, Tianjin 300384, China. E-mail: mfchentj@126.com; tjut_2014@tjut.edu.cn
bSchool of Environmental Science and Safety Engineering, Tianjin University of Technology, Tianjin 300384, China
First published on 29th April 2016
Abstract
Nanocomposites of biodegradable PLLA and magnesium oxide composite (PLLA/MgO-NPs) and surface modified magnesium oxide composite (PLLA/m-MgO-NPs) were prepared using a solution casting method. Effects of the MgO-NPs and m-MgO-NPs on the crystallization behavior and mechanical properties of the PLLA are investigated systematically. Differential scanning calorimetry (DSC) was used to characterize the melting behavior and isothermal crystallization kinetics of pure PLLA and PLLA nanocomposite samples at varying isothermal crystallization temperatures. The Avrami equation was used to calculate the half crystallization time (t1/2) and shown that the m-MgO-NPs is a kind of better nucleating agents than MgO-NPs because it enhanced crystallization rate significantly. Polarized optical microscopy (POM) results showed the density of spherulites increased and their size decreased in PLLA/MgO-NPs and PLLA/m-MgO-NPs samples. While the large amount (1.5 wt%) of MgO-NPs could hinder the crystallization of the PLLA. α′-PLLA emerged more easily than α-PLLA. The nucleation mechanism and geometry of crystal growth of neat PLLA and PLLA nanocomposite materials were determined to be similar. Mechanical property analysis showed both MgO-NPs and m-MgO-NPs could improve greatly the tensile strength, Young's modulus and elongation at break. Especially in the case of PLLA/m-MgO-NPs, the elongation at break was increased by 8.2 times. Uniform dispersion of m-MgO-NPs, and strong interaction and binding force between m-MgO-NPs and the PLLA matrix are favorable for the large enhancement in mechanical properties of the PLLA.
1. Introduction
Poly-L-lactide (PLLA) has attracted wide attention due to its biodegradability and biocompatibility, and has medical applications such as in bone screws, surgical sutures, tissue engineering, and controlled drug delivery.1–3 Although PLLA is suitable for a number of applications, there are several critical shortcomings that restrict its application: severe inflammatory response, relatively poor mechanical properties resulting from its molecular chain structure and low crystallization rate. The PLLA, a semicrystalline polymer material, crystallization properties are important factors affecting its performance. The crystallinity and crystal structure of the polymer influence the thermal properties, mechanical properties, hydrolysis and hydrophilicity, etc.4 Improving the crystallization rate and mechanical properties of PLLA is favorable to process and mold the polymer.5 The crystallization ability of PLLA is poor and the loss of mechanical properties occurs rapidly following initial degradation.6,7 Researchers have shown inorganic nanoparticles act as a heterogeneous nucleating agent and can effectively improve the crystallization ability of PLLA. At the same time, the polarity of the nanoparticles can also increase the hydrophilicity of PLLA.8,9 Therefore, the introduction of inorganic nanoparticles into PLLA has important research value in order to improve crystallization and regulate its hydrolytic and biological behavior.In the previous studies, several inorganic fillers have been added to the PLLA matrix, such as hydroxyapatite (HAP),9 silica nanoparticles,10 titanium dioxide,11 carbon nanotubes,12 and many promising results have be obtained. However, the impacts of these fillers on human body are unclear in situations where they cannot fully biodegrade. Magnesium and its oxide are non-toxic and harmless to human body.13–15 Magnesia nanoparticles are selected as the inorganic filler based on the following reasons. First, magnesia nanoparticles are alkaline oxide, which can act as a buffer to reduce the inflammatory reaction by neutralizing the acidic by-product of PLLA degradation. Second, magnesium is an essential element of human body and magnesium ion is nontoxic.
Fuqiu Ma16 fabricated nanocomposites based on biodegradable PLLA by the incorporation of surface modified magnesia oxide (MgO) nanoparticles. MgO nanoparticles were grafted through ring opening polymerization of ε-caprolactone (ε-CL) using stannous octanoate as a catalyst. The mechanical properties, biodegradability, and protein adhesion behavior of the grafted MgO (g-MgO)/PLLA nanocomposites were investigated by tensile test and in vitro degradation tests. In this study the nanocomposites toughness was decreased. In the study of Chang Hun Kum17,18 et al., the advantages of PLLA using a stereocomplex structure were addressed, where oligo-lactic acid-grafted magnesium hydroxides (MgO-OLA) were synthesized by ring opening polymerization. The structure, morphology, pH change, thermal and mechanical properties, in vitro cytotoxicity, and inflammation effects of the MgO-OLAs and their PLLA composites were evaluated. The tensile strength and modulus of PLLA/Mg80-OLA20 (0–20 wt%) were higher than those of PLLA/magnesium hydroxide. It was also effective in neutralizing the acidic environment caused by the degradation by-product of the PLLA matrix. The incorporation of Mg-OLAs into the PLLA matrix could reduce the inflammatory response of hybrid PLLA. The above research shown MgO particles could improve the tensile strength, Young's modulus, crystallization rate and biocompatibility of the PLLA to a certain extent, but agglomeration occurred when a certain amount of MgO particles were added in the PLLA matrix. That is, excellent dispersion of MgO particles could not be found in the PLLA matrix. Relatively weak interaction and binding force between modified MgO particles and PLLA matrix resulted in a limited improvement in the mechanical properties of PLLA/MgO particles composite.
The malic acid with one hydroxyl and two carboxyl groups, is a naturally occurring organic compound in the tricarboxylic acid cycle of the glucose metabolism in the human body,19 being used as a food additive. Its polymer, poly(malic acid), is well known as a water-soluble, biodegradable, and bio-adsorbable,20,21 and can be modified through its pendent carboxyl groups which can easily react with other bioactive group.22–25 Poly(L-lactic acid-co-malic acid) (PLMA), a functionalized biodegradable copolymer was synthesized and proved to show a better cell adhesion and cell affinity,26 but a lower crystallinity (with a higher fraction of amorphous phase) and more fast degradation rate than the neat PLLA.27 It was reported that a magnetite composite nanoparticle coated by PLMA–fluorescein isothiocyanate was successfully prepared and it is an excellent magnetic-fluorescent tracking agent for stem cell labeling.28
In this work, according to a simple method reported previously,28 the MgO-NPs, L-lactic acid (or oligo-(L-lactic acid)) and malic acid were heated in the vacuum to fabricate the m-MgO-NPs, in which MgO-NPs are grafted by a copolymer, poly(L-lactic acid-co-malic acid) (PLMA). The obtained m-MgO-NPs were expected to increase the crystallization rate and improve the mechanical properties of the PLLA. The PLLA/m-MgO-NPs films were prepared by a solution casting method. For comparison, the neat PLLA and PLLA/MgO-NPs films were also prepared using the same method. Effects of MgO-NPs and m-MgO-NPs on morphology, non-isothermal and isothermal crystallization behaviors, and mechanical properties of the composites were investigated by polarized optical microscopy (POM), differential scanning calorimetry (DSC), and tensile tests.
2. Materials and methods
2.1 Preparation and modification of MgO-NPs
Analytical grade MgCl2·6H2O, C2H2O4·2H2O, cetyl trimethyl ammonium bromide (CTAB) and lactic acid were purchased from Sigma-Aldrich Co. and used as received. MgCl2·6H2O (76.24 g) was dissolved in 250 mL deionized water and 1 mL CTAB (1.0 wt%) was added dropwise. This solution was stirred for 20 min at 40 °C. H2C2O4·2H2O (23.64 g) was added to this solution and the mixture was allowed to react for 20 min. The suspension was collected and centrifuged at 7000 rpm for 10 min. The MgO-NPs precipitate was dried for 1 h in a vacuum oven (85 °C) and sintered in a muffle furnace for 5 h at 600 °C.Malic acid, oligo-L-lactic acid and MgO-NPs (weight ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15) were added to a flask and stirred at 145 °C for 8.5 h under vacuum conditions. The surface modified nanoparticle product, m-MgO-NPs, was washed 3 times in ethyl acetate in an ultrasonic bath and dried to a powder in a vacuum oven at 45 °C for 1 day.
0.15) were added to a flask and stirred at 145 °C for 8.5 h under vacuum conditions. The surface modified nanoparticle product, m-MgO-NPs, was washed 3 times in ethyl acetate in an ultrasonic bath and dried to a powder in a vacuum oven at 45 °C for 1 day.
2.2 Preparation of PLLA/MgO-NPs and PLLA/m-MgO-NPs
PLLA (Mw = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) was purchased from Jinan-Daigang Biotechnology Co. Ltd., China. Before use, PLLA was dried under vacuum at 40 °C for 24 h. The nanocomposites of PLLA/MgO were prepared by stirring PLLA and the MgO-NPs (or m-MgO-NPs) at various weight ratios (0.5, 1.0, and 1.5 wt%) in dichloromethane. After evaporation of dichloromethane, the nanocomposites film was dried overnight in a 45 °C vacuum oven.
000 Da) was purchased from Jinan-Daigang Biotechnology Co. Ltd., China. Before use, PLLA was dried under vacuum at 40 °C for 24 h. The nanocomposites of PLLA/MgO were prepared by stirring PLLA and the MgO-NPs (or m-MgO-NPs) at various weight ratios (0.5, 1.0, and 1.5 wt%) in dichloromethane. After evaporation of dichloromethane, the nanocomposites film was dried overnight in a 45 °C vacuum oven.
2.3 Characterization of composites
3. Results and discussion
3.1 Grafted copolymer amount measurement
Thermogravimetric analysis (TGA) was used to measure grafted polymer amount on the surface of m-MgO-NPs and results are shown in Fig. 2. For the MgO-NPs (red curve), slight weight loss (4%) can be observed from 300 to 450 °C, probably attributed to thermal degradation of unknown impurity. For the m-MgO-NPs (black curve), 3 obvious weight losses occurred at 100 °C (a), 300–350 °C (b) and 400–500 °C (c). The weight loss at 100 °C maybe ascribed to the water evaporation of the m-MgO-NPs. During the fabrication of m-MgO-NPs, only some amount of copolymer (poly(L-lactide-co-malic acid), PLMA) can be successfully grafted on the surface MgO-NPs (grafted coating), while other PLMA just cover loosely the MgO-NPs without grafting (loose coating). Therefore, the weight loss occurred at 300–350 °C (b temperature range) is probably ascribed to thermal degradation of loose coating (PLMA), but that at 400–500 °C (c temperature range) should be thermal degradation of grafted PLMA. From Fig. 2, the weight loss occurred in the (c) temperature range is about 27%, that is, grafted PLMA amount is 27%.Morphologies of MgO-NPs and m-MgO-NPs are presented in ESI (Fig. S1 and S2†). From Fig. S1,† it can be clearly seen that the size of MgO-NPs is about 80 nm with agglomeration of MgO nanoparticles. From Fig. S2,† it seems that the dark m-MgO-NPs with about 120 nm are coated by a viscous layer, which should be the PLMA layer.
3.2 Non-isothermal crystallization and melting behaviors
During the practical processing procedure, articles usually experienced the non-isothermal crystallization process and therefore, it is very important to investigate the non-isothermal crystallization behaviors of semi-crystalline polymers. The non-isothermal crystallization and melting behavior of neat PLLA and PLLA-based nanocomposites were investigated by DSC. In the cooling scans (Fig. 3a), no exothermic peak was observed for neat PLLA sample. The crystallization peaks in PLLA-based nanocomposite samples showed relatively higher intensity, with exception of PLLA/1.5 wt% MgO-NPs. The intensity of the exothermic peak was affected by the concentration and surface modification of the MgO nanoparticles. The accelerated non-isothermal crystallization process observed in PLLA containing 0.5 wt% MgO-NPs was shown to decrease as the concentration of MgO-NPs increased. The m-MgO-NPs concentration did not affect the non-isothermal crystallization temperature and the intensity of the exothermic peak increased with m-MgO-NPs concentration.The crystallization capability of neat PLLA was improved by the addition of MgO nanoparticles, as observed by the pronounced crystallization peak. The enhanced crystallinity in samples containing MgO was probably ascribed to the distribution of nanoparticles. The heterogeneous nucleation effect of the MgO-NPs promoted the crystallization of PLLA in the presence of a small amount (0.5 wt%) of MgO-NPs. As the concentration of MgO-NPs increased, the MgO-NPs were inclined to agglomerate together and became difficult to distribute evenly in the PLLA matrix. The interaction of MgO-NPs may hinder the adjustment of the PLLA molecular chains and the crystallization process. m-MgO-NPs presented a less obvious effect on accelerated crystallization of PLLA compared to the 0.5 wt% MgO-NPs, as evidenced by a less intense crystallization peak. However, for the PLLA/m-MgO-NPs samples, the intensity of crystallization peak of the PLLA increased with the concentration of m-MgO-NPs, probably suggesting that strong bonding between the PLLA and m-MgO-NPs and uniform dispersion of m-MgO-NPs in the PLLA matrix.
The subsequent heating curves are shown in Fig. 3b. For neat PLLA, the glass transition at 60.5 °C (Tg), exothermic peak attributed to the cold crystallization of PLLA at 133.4 °C (Tcc), and endothermic peak attributed to the melting of PLLA at 179.6 (Tm), are observed. As shown in Fig. 3a, neat PLLA in its non-isothermal crystallization process does not crystallize. Therefore, the observed endothermic Tm peak originates from the fusion of PLLA crystallites induced during the subsequent heating process through the cold crystallization at 133.4 °C. For the PLLA-based nanocomposites, it can be seen that all observed transitions are dependent on the concentration of MgO-NPs and m-MgO-NPs. Tg and Tm of the PLLA/MgO-NPs and PLLA/m-MgO-NPs samples are similar to neat PLLA, while the Tcc shifts to lower temperatures, further indicating that MgO nanoparticles facilitate the crystallization of the PLLA indeed (in the heating process, crystallization occurs in lower temperature). With increasing concentrations of the nanoparticle, the Tcc decreases and intensity of Tcc peak of the PLLA remains stable in the case of PLLA/m-MgO-NPs, and the Tcc and intensity of Tcc peak of the PLLA increases in the case of PLLA/MgO-NPs. It reveals that small amount of MgO-NPs is favorable for the crystallization of the PLLA, but large amount of MgO-NPs suppresses the crystallization of the PLLA. In the case of PLLA/m-MgO-NPs, the crystallization ability of the PLLA increases with the loading of the m-MgO-NPs, in good agreement with results in Fig. 3a.
3.3 Isothermal crystallization and melting behaviors
In order to fully understand the effect of MgO-NPs and m-MgO-NPs on the bulk crystallization rate of the PLLA, isothermal crystallization and subsequent melting behaviors of neat PLLA (Fig. 4) and the PLLA-based nanocomposites (PLLA/MgO-NPs and PLL/m-MgO-NPs) (Fig. 5) were investigated using DSC. The shift of exotherm peaks to longer times with increasing Tc indicates a decrease in crystallization rate for neat PLLA, with exception of 110 °C at which crystallization is most fast (Fig. 4a), in good agreement with previous report.30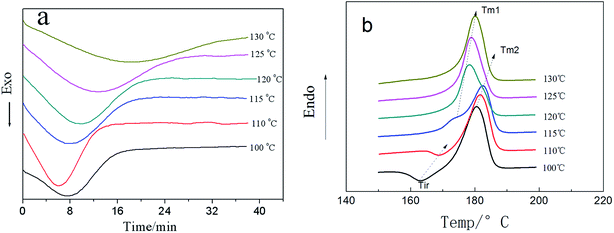 | ||
| Fig. 4 DSC isothermal crystallization curves (a) and subsequent melting curves (b) of neat PLLA at different isothermal crystallization temperatures. | ||
 | ||
| Fig. 5 DSC isothermal crystallization curves at Tic = 110 °C (a) and subsequent melting (b) of neat PLLA and PLLA-based nanocomposites. | ||
At Tc = 110 °C (Fig. 5a), the crystallization time of the PLLA/MgO-NPs (with the exception of PLLA/1.5 wt% MgO-NPs) and PLLA/m-MgO-NPs were shorter than that of neat PLLA. In the case of PLLA/0.5 wt% MgO-NPs, PLLA takes the shortest time to complete its crystallization, while 1.5 wt% MgO-NPs retards the crystallization of the PLLA, where the crystallization time is even longer than that of neat PLLA. For brevity, 110 °C as an example are presented to describe the isothermal crystallization and subsequent melting behavior of PLLA-based nanocomposites, and other isothermal crystallization temperatures have also been investigated and similar crystallization behaviors were obtained.
The subsequent melting behaviors of neat PLLA were analyzed and the corresponding DSC heating curves are shown in Fig. 4b. Yasuniwa et al. reported that when the Tc is between 113 °C and 135 °C, α′ and α forms coexist as a crystalline polymorph. Tm1 is related to the fusion of primary crystallites (α form crystal) formed during the isothermal crystallization process, while Tm2 to the fusion of new crystallites formed during the DSC heating process through the melt–recrystallization (the transformation from α′ to α)–melt process of primary crystallites. Exotherm peak (Tir) can be found at the right side of melting peak in the case of 100 and 110 °C, assigned to the recrystallization in the heating process, indicating that small amount of the PLLA molecular chain needs rearrangement to reach a more perfect crystallization.
For the PLLA-based nanocomposite, only the sample obtained after being isothermally crystallized at 120 °C was analyzed and the DSC heating curves are shown in Fig. 5b. The degree of crystallinity (Xc) of the PLLA is calculated according to the following equation:
| Sample | Xt/100% | k/min−1 | n | t1/2/min |
|---|---|---|---|---|
| Neat PLLA | 31.14 | 1.76 × 10−2 | 2.80 | 8.1 |
| 0.5% MgO | 42.53 | 2.84 × 10−1 | 2.79 | 1.9 |
| 1% MgO | 38.66 | 4.12 × 10−2 | 2.92 | 3.8 |
| 1.5% MgO | 30.17 | 9.64 × 10−3 | 3.12 | 9.2 |
| 0.5% m-MgO | 36.77 | 3.86 × 10−2 | 2.89 | 4.1 |
| 1% m-MgO | 35.92 | 4.21 × 10−2 | 3.08 | 3.7 |
| 1.5% m-MgO | 35.04 | 4.05 × 10−2 | 2.98 | 3.9 |
3.4 Isothermal crystallization kinetics
The relative crystallinity (Xt) as a function of crystallization time (t) is shown in Fig. 6. Xt is calculated as follows:The isothermal crystallization kinetics of semi-crystalline polymers can be described by the Avrami relation:
| 1 − X(t) = exp[−k(T)tn] |
The formula can also be expressed:
lg{−ln[1 − X(t)]} = lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k + n k + n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) t t |
Half-time of crystallization (t1/2) is defined as the time when Xt is equal to 0.5 and is calculated as follows:
t1/2 = (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/k)1/n 2/k)1/n |
The values of lg{−ln[1 − X(t)]} plotted versus lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) t, shown in Fig. 7. The Avrami exponent n and the crystallization rate constant k were calculated from the slope and intercept of the linear fit. The Avarmi equation rarely describes the whole crystallization process and is typically valid at the initial crystallization stage. The linear portions of the plots and half-time of crystallization results are summarized in Tables 1 and S1† (crystallization kinetic parameters of the neat PLLA).
t, shown in Fig. 7. The Avrami exponent n and the crystallization rate constant k were calculated from the slope and intercept of the linear fit. The Avarmi equation rarely describes the whole crystallization process and is typically valid at the initial crystallization stage. The linear portions of the plots and half-time of crystallization results are summarized in Tables 1 and S1† (crystallization kinetic parameters of the neat PLLA).
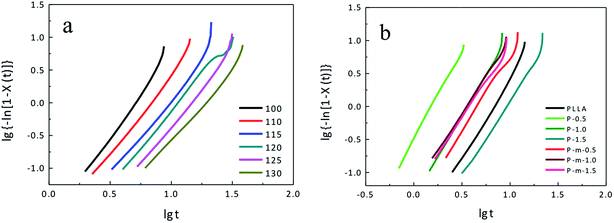 | ||
| Fig. 7 Avrami plots for (a) neat PLLA and (b) PLLAs containing different concentrations of MgO-NPs or m-MgO-NPs. | ||
From Fig. 6a, it takes the shortest time to reach 100% relative crystallinity for neat PLLA melt-crystallized at 110 °C. For neat PLLA isothermally crystallized at other temperatures (100, 115, 120, 125 and 130 °C), crystallization time increases with temperature when it reaches 100% relative crystallinity. From Fig. 6b, the time at which 100% relative crystallinity is achieved, is in the following order: 1.5 wt% MgO-NPs > neat PLLA > 0.5 wt% m-MgO-NPs > 1.5 wt% m-MgO-NPs > 1 wt% MgO-NPs ≈ 1 wt% m-MgO-NPs > 0.5 wt% MgO-NPs.
The plots in Fig. 7 are linear for all composites at the initial crystallization stage, indicating that the isothermal crystallization kinetics of neat PLLA and PLLA-based composites can be well described by the Avarmi equation. The order of time used for reaching 100% relative crystallinity in Fig. 7 is in good agreement with that in Fig. 6. As mentioned above, the Avrami exponent, n, relates to the mode of nucleation and the dimensionality of the growing crystals. For neat PLLA melt-crystallized at various temperatures, all the n values are close to 3 (Table S1†). It seems that neat PLLA grows in 2-dimensional manner, is not in agreement with result of Fig. 8 (image of neat PLLA spherulite). We consider that some unknown impurities in neat PLLA probably result in heterogeneous nucleation of the PLLA. Therefore, neat PLLA grows in 3-dimensional manner. For PLLA-based composites, their n values are also close to 3 (Table 1), suggesting that heterogeneous nucleation mechanism and a three-dimensional crystallization growth of the PLLA.
 | ||
| Fig. 8 Polarized optical microscopy images of neat PLLA, PLLA/MgO-NPs and PLLA/m-MgO-NPs melt-crystallized at 120 °C. Scale bar is 100 μm. | ||
Low k value and high t1/2 value is two indicatives of slow crystallization. The order of crystallization rate evidenced by k value and t1/2 value in Tables S1† and 1 is also in good agreement with that in Fig. 6 and 7. From Table 1, t1/2 of the PLLA with 0.5 wt% MgO-NPs is 1.9 min, significantly lower than that of neat PLLA (8.1 min) and the PLLA with 0.5 wt% MgO-NPs (9.2 min), suggesting that 0.5 wt% MgO-NPs exhibits best nucleating effect. As mentioned above, 1.5 wt% MgO-NPs were difficult to disperse evenly in the PLLA matrix and poorly distributed MgO-NPs are predicted to decrease the crystallization rate.
3.5 Effect of MgO-NPs and m-MgO-NPs on spherulite size and the mechanical properties of PLLA
The effects of MgO-NPs and m-MgO-NPs nucleation agents on the spherulitic morphology of the PLLA were observed by polarized optical microscopy (POM). POM images of neat PLLA and PLLA/MgO-NPs and PLLA/m-MgO-NPs melt-crystallized at 120 °C (complete crystallization), are shown in Fig. 8. Neat PLLA exhibits sporadic, regular and larger spherulites with typical Maltese-Cross appearance. After incorporation of MgO-NPs (0.5% and 1%) and m-MgO-NPs, the size of the PLLA spherulite decreases and the density increases, suggesting that MgO-NPs (0.5% and 1%) and m-MgO-NPs exhibit insignificantly excellent nucleating effect on the crystallization of PLLA, because the heterogeneous nucleating agent provide the nucleation sites on which the PLLA crystals grow. However, 1.5% MgO-NPs enhance the size of the PLLA spherulite and decrease the density of the PLLA spherulite, indicating that much amount of MgO-NPs hinders the adjustment of the PLLA molecular chains because the agglomeration of much amount of MgO-NPs is unfavorable for the uniform dispersion of MgO-NPs. Due to the strong interaction and binding force between m-MgO-NPs and the PLLA matrix, uniform dispersion of m-MgO-NPs occurs, resulting in excellent dispersion of m-MgO-NPs in the PLLA matrix. The POM results are in good agreement with the DSC results.The mechanical strength of neat PLLA and PLLA-based composites are presented in Fig. 9 and S3 in ESI,† respectively. From two figures, the tensile strength, Young's modulus and elongation at break of the PLLA are significantly increased after addition of MgO-NPs and m-MgO-NPs. Generally, with incorporating the same amount of nanoparticle, the tensile strength of PLLA/MgO-NPs is slightly higher than that of PLLA/m-MgO-NPs (Fig. 9a and S3†), may ascribed to higher crystallinity degree of the PLLA in the case of PLLA/MgO-NPs, as shown in Table 1. Young's modulus of the PLLA with small amount of MgO-NPs (0.5 wt%) is higher than that with 0.5 wt% m-MgO-NPs. While with further addition of nanoparticles (1 and 1.5 wt%), Young's modulus of the PLLA with MgO-NPs is slightly lower than that with m-MgO-NPs. It is noted that, the elongation at break of PLLA/m-MgO-NPs is much greater than that of the corresponding PLLA/MgO-NPs, probably associated with uniform dispersion of m-MgO-NPs in the PLLA matrix and strong interaction between m-MgO-NPs and the PLLA molecular chain. The above results reveal that the mechanical properties of the PLLA is greatly improved via incorporating inorganic MgO-NPs and inorganic/organic composite m-MgO-NPs. Especially for the PLLA with the brittleness, the elongation at break is increased by 8.2 times upon addition of 1.5% m-MgO-NPs with a large enhancement in the tensile strength and Young's modulus, so m-MgO-NPs is an ideal nucleating agent in improving mechanical properties of the PLLA.
 | ||
| Fig. 9 Tensile strength (a), Young's modulus (b) and elongation at break (c) of neat PLLA, PLLA/MgO-NPs and PLLA/m-MgO-NPs. | ||
The mechanical properties of the composites are affected by the dispersion of reinforcement in the matrix and the interaction between the reinforcement and the matrix. As discussed previously, the surface modification improved the nanoparticles dispersion in the PLLA matrix. Mechanism of large enhancement in mechanical properties of the PLLA in the presence of m-MgO-NPs is proposed in Fig. 10. The yellow and blue balls represent a MgO nanoparticle (MgO-NP) and PLLA matrix (or PLLA spherulite), respectively. Poly(L-lactic acid-co-malic acid) (PLMA) is grafted on the surface of the MgO-NPs to form the m-MgO-NPs. Via the interaction and binding force between the m-MgO-NPs and PLLA matrix, m-MgO-NPs can dispersed in the PLLA matrix in the following three forms: intergranular, inter-chip and between molecular chains (as denoted in Fig. 10), leading to excellent and uniform dispersion of the m-MgO-NPs.
 | ||
| Fig. 10 Schematic illustration for dispersion of m-MgO-NP and interaction between m-MgO-NP and PLLA matrix for enhancing mechanical properties. | ||
The nanocomposite may be effective on avoiding the external stress and maintain high strength due to inter-molecular chain crosslink of PLMA and PLLA, increasing the binding force between the nanoparticles and the substrate. Thereby the PLLA/m-MgO-NPs could meet the requirements of clinical applications such as internal fracture fixation by maintaining a dramatic strength and plasticity in the case of lower crystallinity. Research on effect of m-MgO-NPs on anti-inflammation of the PLLA is in progress in our group and relevant work will be reported later.
4. Conclusions
The effects of MgO-NPs and m-MgO-NPs on the crystallization behavior and mechanical properties of PLLA were studied. DSC results suggest that small amount (0.5 wt%) of MgO-NPs greatly increased the crystallization rate and spherulitic density, and decreased spherulitic size of the PLLA, but large amount of MgO-NPs could hinder the crystallization of the PLLA and suppress the adjustment of the PLLA molecular chains, due to agglomeration of much amount of MgO-NPs. m-MgO-NPs also increased the crystallization to a certain extent, although its nucleating affect is less obvious than that of 0.5 wt% MgO-NPs. Due to excellent and uniform dispersion of m-MgO-NPs in the PLLA matrix, and strong interaction and binding force between m-MgO-NPs and the PLLA matrix, mechanical properties of the PLLA were significantly increased.Acknowledgements
The authors acknowledge the financial support for this work from the National Nature Science Foundation of China (No. 51371126 & 21304070), Science and Technology supporting program in Tianjin (No. 14ZCZDGX00007), Major science and technology projects in Tianjin (No. 15ZXQXSY00080) Tianjin Natural Science Foundation (No. 15JCYBJC47300) and Science and Technology developing Foundation of Tianjin High Education (No. 20110301).References
- V. Grigoriou, I. M. Shapiro, E. A. Cavalcanti-Adam, R. J. Composto, P. Ducheyne and C. S. Adams, J. Biol. Chem., 2005, 280, 1733–1739 CrossRef CAS PubMed.
- A. J. R. Lasprilla, G. A. R. Martinez, B. H. Lunelli, A. L. Jardini and R. M. Filho, Biotechnol. Adv., 2012, 30, 321–328 CrossRef CAS PubMed.
- I. Armentano, N. Bitinis, E. Fortunati, S. Mattioli, N. Rescignano, R. Verdejo, M. A. Lopez-Manchado and J. M. Kenny, Prog. Polym. Sci., 2013, 38, 1720–1747 CrossRef CAS.
- C. H. Kum, Y. Cho, S. H. Seo, Y. K. Joung, D. J. Ahn and D. K. Han, Small, 2014, 10, 3783–3794 CrossRef CAS PubMed.
- B. Na, N. Tian, R. Lv, S. Zou and W. Xu, Macromolecules, 2010, 43, 1156–1158 CrossRef CAS.
- J. M. Anderson, A. Rodriguez and D. T. Chang, Semin. Immunol., 2008, 20, 86–100 CrossRef CAS PubMed.
- Z. Hong, P. Zhang, C. He, X. Qiu, A. Liu, L. Chen, X. Chen and X. Jing, Biomaterials, 2005, 26, 6296–6304 CrossRef CAS PubMed.
- L. T. Lim, R. Auras and M. Rubino, Prog. Polym. Sci., 2008, 33, 820–852 CrossRef CAS.
- H. Tsuji, K. Shimizu and Y. Sato, J. Appl. Polym. Sci., 2012, 125, 2394–2406 CrossRef CAS.
- J. Zhang, J. Lou, S. Ilias, P. Krishnamachari and J. Yan, Polymer, 2008, 49, 2381–2386 CrossRef CAS.
- I. H. Kim and Y. G. Jeong, J. Polym. Sci., Part B: Polym. Phys., 2010, 48, 850–858 CrossRef CAS.
- H. M. Chen, C. X. Feng, W. B. Zhang, J. Yang, T. Huang, N. Zhang and Y. Wang, Polym. Degrad. Stab., 2013, 98, 198–208 CrossRef CAS.
- R. Giorgi, C. Bozzi, L. Dei, C. Gabbiani, B. W. Ninham and P. Baglioni, Langmuir, 2005, 21, 8495–8501 CrossRef CAS PubMed.
- H. Yan, X. H. Zhang, L. Q. Wei, X. Liu and B. Xu, Powder Technol., 2009, 193, 125–129 CrossRef CAS.
- K. J. Jeon, H. R. Moon, A. M. Ruminski, B. Jiang, C. Kisielowski, R. Bardhan and J. J. Urban, Nat. Mater., 2011, 10, 286–290 CrossRef CAS PubMed.
- F. Ma, X. Lu, Z. Wang, Z. Sun, F. Zhang and Y. Zheng, J. Phys. Chem. Solids, 2011, 72, 111–116 CrossRef CAS.
- C. H. Kum, Y. Cho, Y. K. Joung, J. Choi, K. Park, S. H. Seo, Y. S. Park, D. J. Ahn and D. K. Han, J. Mater. Chem. B, 2013, 1, 2764–2772 RSC.
- L. T. Lim, R. Auras and M. Rubino, Prog. Polym. Sci., 2008, 33, 820–852 CrossRef CAS.
- T. Kajiyama, H. Kobayashi, T. Taguchi, K. Kataoka and J. Tanaka, Biomacromolecules, 2004, 5, 169–174 CrossRef CAS PubMed.
- M. Vert and R. W. Lenz, Polym. Prepr., 1979, 20, 608–611 CAS.
- Y. Abe, S. Matsumura and K. Imai, Yukagaku, 1986, 35, 937–944 CAS.
- S. Cammas, M. M. Bear, L. Moine, R. Escalup, G. Ponchel, K. Kataoka and P. Guérin, Int. J. Biol. Macromol., 1999, 25, 273–282 CrossRef CAS PubMed.
- B. He, J. Bei and S. Wang, Polym. Adv. Technol., 2003, 14, 645–652 CrossRef CAS.
- T. Kajiyama, H. Kobayashi, T. Taguchi, Y. Komatsu, K. Kataoka and J. Tanaka, Mater. Sci. Eng., C, 2004, 24, 821–825 CrossRef.
- L. Wang, X. Jia, Y. Chen, Y. Che and Z. Yuan, J. Biomed. Mater. Res., Part A, 2008, 87, 459–469 CrossRef PubMed.
- B. He, Y. Wan, J. Bei and S. Wang, Biomaterials, 2004, 25, 5239–5247 CrossRef CAS PubMed.
- B. He, J. Bei and S. Wang, Polymer, 2003, 44, 989–994 CrossRef CAS.
- L. Wang, K. G. Neoh, E. T. Kang, B. Shuter and S. C. Wang, Biomaterials, 2010, 31, 3502–3511 CrossRef CAS PubMed.
- D. Garlotta, J. Polym. Environ., 2001, 9, 63–84 CrossRef CAS.
- P. Pan and Y. Inoue, Prog. Polym. Sci., 2009, 34, 605–640 CrossRef CAS.
- Y. Chen, S. Wang, Q. Chen, Z. Xi, C. Wang, X. Chen, X. Feng, R. Liang and J. Yang, Eur. Polym. J., 2015, 72, 222–237 CrossRef CAS.
- J. Yang, Y. Chen, Q. Song, J. Liu, C. Bi, R. Liang, T. Dong and X. Feng, Ind. Eng. Chem. Res., 2015, 54, 8048–8055 CrossRef CAS.
- J. Yang, Y. Chen, L. Hua, R. Liang and D. Zhu, J. Appl. Polym. Sci., 2016, 133, 42957–42964 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra05514a |
| This journal is © The Royal Society of Chemistry 2016 |






