Ultrasound mediated, green innovation for the synthesis of polysubstituted 1,4-dihydropyridines†
Abstract
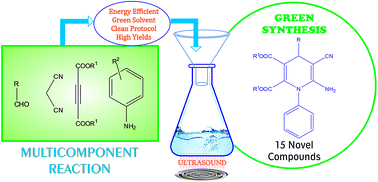
An elegant, atom efficient protocol via a one-pot four-component cyclocondensation reaction of aromatic aldehydes, malononitrile, acetylenedicarboxylates and arylamines catalyzed by copper(I) iodide in aqueous medium under ultrasound irradiation has been developed for the synthesis of a series of novel pharmacologically interesting polysubstituted 1,4-dihydropyridines. In comparison with the reported methods, our approach is expedient and offers several advantages such as: a shorter reaction time, excellent yields, milder conditions, convenience and environmental benignity. We have herein successfully demonstrated the utility of sonication in a multicomponent reaction (MCR), which exhibits a better functional group tolerance, and straightforward product isolation and purification by precipitation.

 Please wait while we load your content...
Please wait while we load your content...