Influence of a surface modified Li anode on the electrochemical performance of Li–S batteries
Meifen Wu,
Jun Jin and
Zhaoyin Wen*
CAS Key Laboratory of Materials for Energy Conversion, Shanghai Institute of Ceramics, Chinese Academy of Sciences, Shanghai, 200050, China. E-mail: zywen@mail.sic.ac.cn; Fax: +86 21 52413903; Tel: +86 21 52411704
First published on 13th April 2016
Abstract
A bifunctional protective film is successfully prepared on Li metal surface by hexamethylditin modification in order to migrate the corrosion of sulfur-containing species and other electrolyte components. XRD, XPS, CV, SEM, AC impedance, polarization measurements and galvanostatic charge/discharge cycling tests are applied to characterize the film. The experimental results show that the protective film is dense and homogenous with a thickness of ∼10 nm. And under the protection of the film, the modified Li anode exhibits higher stability, lower interface resistance and a lower polarization potential difference than those of the as-received Li anode. Moreover, the cycling performance and rate capability of a Li/S cell are greatly improved by surface modification of the Li anode, delivering a discharge capacity of ∼800 mA h g−1 and a coulombic efficiency of more than 98.5% over 150 cycles at 0.2C as well as a notable rate performance with 510 mA h g−1 at 5C. After cycling, fewer sulfur-containing species are found on the modified Li anode surface than on the as-received Li anode surface, indicating the effective protection of the Li anode.
Introduction
The increasing demand of high energy devices for applications in electric vehicles (EV), hybrid EV (HEV) and grid-scale stationally energy storage systems has created an increase in research on advanced rechargeable lithium batteries.1,2 Among the various battery systems, the lithium–sulfur (Li–S) battery has attracted tremendous attention due to its high theoretical energy density (∼2567 W h kg−1), and the natural abundance, non-toxicity and low cost of sulfur.3–5 However, several notable problems, such as fast capacity degradation on cycling, low coulombic efficiency6,7 and severe self-discharge,8–10 have limited its practical realization, which can be mainly attributed to the formation of high-order polysulfides (S82−, S62−, S42−) during the discharge–charge process.11 The high-order polysulfides are highly soluble in organic electrolyte and could easily migrate throughout the separator to be reduced by Li, forming solid precipitates (Li2S2 and/or Li2S) on Li surface.12–14 The continuous deposition of insoluble lithium sulfides may seriously hinder the rapid access of Li ion, and cause poor rate capability.2,5,15 Moreover, for lithium-based batteries, one inevitable problem lies in the growth of lithium dendrites, which origenates from an inhomogeneous deposition of Li and can cause safety problems of the Li–S batteries.16 In addition, the high reactivity of Li with the electrolytes leads to the formation of a solid electrolyte interphase (SEI) layer, causing a remarkable irreversible capacity loss and low deposition efficiency of Li upon charging.17–20 For a practical Li–S battery, this condition will require an excess amount of Li to pair with S cathode in order to achieve high energy output of the system.21 Considering of the above issues, the protection of lithium anode is a quite effective strategy for the electrochemical performance improvement of Li–S battery.Strong efforts have been devoted to explore the suitable surface modifications to protect the Li anode. One effective approach is to introduce a thin and stable passivation layer on Li surface through adding electrolyte additives, such as LiNO3,22–25 P2S5,26 vinylene carbonate,27 selected cations (Cs+, Rb+).28 Another effective strategy is to make a physical barrier (film) on Li surface by using Li alloy passivation layer,29 polymer coatings30–32 and solid electrolytes.33,34 In addition, some alternative anodes have also been used, such Li–B alloy anode, which can effectively prevent the lithium dendrite growth and enhance cycle performance and security of Li–S cells.35,36 All these attempts have achieved some progress, but could not solve all the problems at the same time.
In this work, we introduce hexamethylditin ((CH3)3SnSn(CH3)3) as a new functionalization agent to modify Li surface by reacting with lithium and producing a protective film of (CH3)3Sn–Li, which is expected to have bifunctional protection effect of Li–Sn alloy and organic coating. Such protective film will have enough density to isolate the lithium sulfides and good Li+ ion conductivity for Li+ ion transportation under electrical field. Moreover, it can be easily realized on Li anode ex situ before assembling the batteries. The electrochemical property of the modified Li metal electrode is systematically investigated in a Li/S battery test system, which shows an obvious and effective effect in mitigating Li corrosion, thus improving the cell performance. The possible mechanism is also explored and discussed.
Experimental section
The protection of the Li metal anode
The protective film was prepared according to the synthesis process of stannly-lithium ((CH3)3Sn–Li) in the ref. 37. First, lithium metal foils (China Energy Lithium Co., 450 μm) were received and stored in an argon atmosphere glove-box with both H2O and O2 lower than 1 ppm. Then, 5 mol% naphthalene (Sigma-Aldrich, 99%) (calculated on the base of hexamethylditin) was added in 0.1 M hexamethylditin ((CH3)3SnSn(CH3)3, Sigma-Aldrich, 99.9%)/anhydrous tetrahydrofuran (THF, Sigma-Aldrich, 99.9%) solution and stirred homogenously to get a mixed solution. The Li foil was immersed in the mixed solution and stirred vigorously for 3 min. Finally, the coated lithium samples were taken out and allowed to dry in the glove-box.Preparation of sulfur cathode
The S/C composite was prepared by a melting-diffusion strategy. Certain amount of sulfur powder (Sigma-Aldrich) was mixed with Ketjen black (KB) (Akzo Nobel Corp.) in the weight ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 by ball milling, then the mixture was placed in a crucible and heated at 155 °C for 12 h under vacuum condition. To make a cathode, 80% S/C composite, 5 wt% acetylene black (AB), 5 wt% multi-wall carbon nanotubes, 5 wt% carboxy methyl cellulose (CMC) and 5 wt% (styrene–butadiene rubber) SBR were mixed in deionized water to form a homogeneous slurry by ball milling. Then the slurry was cast onto aluminum foil substrate by tape casting technique. After the water was evaporated, the electrode was cut into discs of 14 mm diameter and then dried at 60 °C under vacuum for 12 h. Accordingly, sulfur loading in the cathode was around 1 mg cm−2. CR2025 type coin cells were assembled in a glove box with Celgard 2320 as the separator and Li foils as both the counter and reference electrodes. The electrolyte consisted of 1 M lithium bis(trifluoromethanesulfonyl) (LiTFSI) in a mixture of 1,3-dioxolane (DOL) and dimethyl ether (DME) (1
4 by ball milling, then the mixture was placed in a crucible and heated at 155 °C for 12 h under vacuum condition. To make a cathode, 80% S/C composite, 5 wt% acetylene black (AB), 5 wt% multi-wall carbon nanotubes, 5 wt% carboxy methyl cellulose (CMC) and 5 wt% (styrene–butadiene rubber) SBR were mixed in deionized water to form a homogeneous slurry by ball milling. Then the slurry was cast onto aluminum foil substrate by tape casting technique. After the water was evaporated, the electrode was cut into discs of 14 mm diameter and then dried at 60 °C under vacuum for 12 h. Accordingly, sulfur loading in the cathode was around 1 mg cm−2. CR2025 type coin cells were assembled in a glove box with Celgard 2320 as the separator and Li foils as both the counter and reference electrodes. The electrolyte consisted of 1 M lithium bis(trifluoromethanesulfonyl) (LiTFSI) in a mixture of 1,3-dioxolane (DOL) and dimethyl ether (DME) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) containing 1 wt% lithium nitrate (LiNO3). The sulfur/electrolyte ratio was about 22.5 g mL−1. The symmetrical Li cells were prepared with Li metal as both cathode and anode, and the same electrolyte and separator.
1, v/v) containing 1 wt% lithium nitrate (LiNO3). The sulfur/electrolyte ratio was about 22.5 g mL−1. The symmetrical Li cells were prepared with Li metal as both cathode and anode, and the same electrolyte and separator.
Characterization
Scanning electron microscope (SEM, HITACHI, S-3400N) and X-ray photoelectron spectroscopy (XPS, Thermo Scientific, ESCALAB 250) were taken to analyze the morphologies and components of the protective film on Li metal electrode surface before cycling, respectively. After charge–discharge cycling test, the Li metal electrode was taken out from the cell and washed by anhydrous DME to remove any electrolyte residuals. Then, the Li metal electrode was sealed in a self-designed air-isolating container and transferred quickly into the SEM equipment under the protection of Ar flow. Finally, the surface morphologies and components of the Li metal electrode after cycling were identified by SEM and energy dispersive spectrum (EDS, HITACHI S-3400).AC impedance measurements were carried out on an Autolab Electrochemical Workstation over the frequency range of 0.01 to 105 Hz with amplitude of 10 mV. The Li+ plating-stripping process of Li/Li cell was measured at a current density of 0.1 mA cm−2 with charge amount of 0.06 C cm−2 for each cycle. The cyclic voltammogram (CV) of Li–S cells was conducted with an Autolab Electrochemical Workstation at a scan rate of 0.2 mV s−1 in a voltage range of 1.8–2.8 V (vs. Li/Li+). The galvanostatic charge–discharge process was performed in a voltage range of 1.8–2.8 V (vs. Li/Li+) at 0.2C (1C = 1675 mA h g−1) with a LAND CT2001A battery test system.
Results and discussion
Fig. 1 depicts the SEM images of Li metal surfaces before and after (CH3)3SnSn(CH3)3 modification at low (a and b) and high magnification (c and d), respectively. As seen in the Fig. 1(a) and (c), the as-received Li surface has a smooth surface expect some white drawbacks, which is caused by the unavoidable interactions between active lithium and air components during preparation process. Hence, a native film consisting of Li2CO3, LiOH, and Li2O is always formed on as-received Li electrode surface, which could affect the quality and performance of any coating layer.38 In this work, the inert solvent THF in the mixed solution not only plays a role of a clean agent to remove the undesired compounds,39 but also provides a reactive environment for (CH3)3SnSn(CH3)3 modification. Also, it allows the lithium surface to dry quickly when removed from the solution. As shown in Fig. 1(b) and (d), a homogenous and dense protective film is fully covered on the Li electrode surface after treated by the mixed solution. | ||
| Fig. 1 SEM images of the surfaces of an as-received Li electrode (a and c) and a surface modified Li electrode (b and d) treated by a mixed solution of 0.1 M (CH3)3SnSn(CH3)3/THF-5 mol% naphthalene. | ||
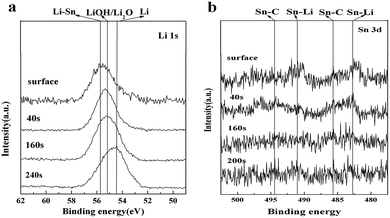
The chemical composition of the film formed on Li electrode surface plays a key role during the charge and discharge of the electrode in the nonaqueous electrolyte. Fig. 2 shows the XPS spectra of Sn 3d and Li 1s for Li electrode after (CH3)3SnSn(CH3)3 surface modification. A strong peak at around 55.8 eV on the surface is attributed to a single bond of Li–Sn, indicating the formation of (CH3)3Sn–Li. As the etching time increases, the most intense peak shifts to a lower binding energy of 54.4 eV, which is ascribed to Li metal as well as some Li2O/LiOH. Two small peaks observed at around 482.6 eV and 485.6 eV are indexed to Sn–Li and Sn–C, respectively, also proving the formation of (CH3)3Sn–Li. Two corresponding peaks at around 491 eV and 494.3 eV are also assigned to the formation of Sn–Li and Sn–C because Sn 3d peak itself comprises of Sn 3d3 peak and Sn 3d5 peak. However, as the atom concentration of Sn in the protective film is only 0.03%, while those of Li, C, O are 42.41%, 21.98%, 35.58%, respectively, it causes the Sn–C and Sn–Li peaks very weak. Moreover, the Sn–C and Sn–Li peaks are disappeared after 200 s, and as the scan rate is 0.05 nm s−1, the thickness of the protective film is estimated to be 10 nm. Based on the above results, we propose that the following reactions occur:
| Li + C10H8 → LiC10H8 (ref. 40 and 41) | (1) |
| 2LiC10H8 →Li2C10H8 + C10H8 (ref. 42) | (2) |
| C10H82− + (CH3)3SnSn(CH3)3 → C10H8 + 2(CH3)3Sn− (ref. 43 and 44) | (3) |
| (CH3)3Sn− + Li+ → (CH3)3SnLi | (4) |
From this reaction scheme, it can be seen that the native film components of Li2O and LiOH do not react with (CH3)3SnSn(CH3)3, meaning that the removal of native components by THF is very important to enhance the homogeneity and density of the protective film.
In order to investigate the interfacial behavior of a modified Li electrode in contact with electrolyte for a long time, Fig. 3(a) and (b) exhibit the time evolution of AC impedance spectra of the cells with as-received Li electrodes and surface-modified Li electrodes, respectively. All spectra are composed of two partially overlapped semicircles in high-middle and low frequency regions, which can be fitted by an equivalent circuit in the inset of Fig. 3(a) with a Nova 1.10 software. Re is bulk resistance of the cell, which corresponds to high frequency intercept at the real axis. The diameter of the large semicircle at high frequency corresponds to the resistance of Li ion migration through the SEI layer (Rf), and the diameter of the small semicircle at medium-low frequency is assigned to the charge-transfer resistance (Rct), which is related to charge-transfer process at the electrode–electrolyte interface (probably across the passivation film).23,25,45 When Rf is much higher than Rct, the semicircle of Rct may not be displayed as evidenced by the Nyquist plot of as-received Li electrode at 72 h. And the Qi (constant phase element) denotes the capacitance of each component to reflect the depressed semicircular shape. The semicircle at low frequency region is expressed by ‘Cothyperbol’ element O, which suggests that a stagnant layer of finite length is formed in the electrolyte for Li+ ion diffusion.
As seen in Fig. 3c, both Rf and Rct values of the cell with as-received Li electrodes continuously increases with time, which can be attributed to the gradual growth of a resistive layer resulting from the reaction between Li electrode and the electrolyte components. However, the Rf and Rct values are increased more slowly and eventually stabilized after 48 h. The constant Rf and Rct indicates that the side reactions between the modified Li electrode and the electrolyte are effectively suppressed, so the interfacial stability of the Li electrode is improved by (CH3)3SnSn(CH3)3 modification. In addition, the low Rct of the cell with modified Li electrode indicates that the protective film has high Li+ ion conductivity, which will be helpful to decrease internal resistance of the cell.
To investigate the stability of the modified Li electrode during Li+ plating/stripping, symmetric cells were cycled at 0.1 mA cm−2 for each plating–stripping cycle for 10 min. Fig. 4(a) and (b) exhibit the overpotential–current–time curves of the cells with different Li electrodes. Compared with the cell with as-received Li electrode, the modified Li electrode has a lower and more stable polarization potential difference, suggesting that Li+ stripping/plating behavior is more stable after the surface modification of Li electrode, which is due to the stable protective film on the modified Li electrode. However, the overpotential of the cell with as-received Li electrode gradually increases during the cycling except a slight decreases in a period of 500–600 min (ca. 50th–60th cycle), indicating that SEI layer of as-received Li has gone through a broken-repaired process during this period of time.46 Moreover, the impedance spectra of the symmetric cells shown in Fig. 4(c) and (d) have also confirmed the polarization test results. The SEI impedance of the half-cell with as-received Li electrode keeps increasing after different cycles, resulting in high polarization voltage. In contrast, the SEI resistance of the half-cell with modified Li electrode is much lower than that of as-received Li, and its value is mainly restrained between 60 ohm and 70 ohm, further improving that the interfacial stability of Li electrode is highly enhanced by (CH3)3SnSn(CH3)3 modification.
The electrochemical characteristics of the Li/S cells with different anodes were investigated by CV between 1.8 V and 2.8 V at a scan rate of 0.2 mV s−1 and the results are presented in Fig. 5(a)–(d). Two pairs of redox peaks appear in the CV profile of both cells, indicating that the surface modification of Li anode show no influence on the electrochemical reactions. In cathodic scan, two distinct reduction peaks of both cells are observed at around 2.3 V and 2 V except the first scan of Li–S cell with modified Li anode, which corresponds to the reduction of element sulfur to soluble polysulfides and then to the insoluble Li2S2/Li2S, respectively.47,48 The reduction peaks in the first scan of Li–S cell with modified Li anode shift to low value and exhibit a wider potential range compared to the other scans, indicating slower kinetics of element sulfur to Li2S2/Li2S. It can be attributed to the increased thickness and density of SEI film on modified Li surface, meaning that an initial activation process is required for the electrochemical reaction of modified lithium and sulfur. It is noticeable that the highly consistent overlap of the cathodic peaks after the first cycle in Fig. 5(b) indicates that the electrochemical process is highly stable with (CH3)3SnSn(CH3)3 modification. In anodic scan of both cells, a clear oxidation peak is observed at around 2.31 V and followed by a shoulder peak around 2.39 V, corresponding to the conversion of lithium sulfide into polysulfides and then to element sulfur. The exact redox potential differences reflecting to the conversion of polysulfides into element sulfur and the transformation between lithium sulfide and polysulfides are recorded by ΔE1 and ΔE2, respectively, and shown in Fig. 5(c). It is noticed that except the first scan, the ΔE2 of the cell with modified Li anode is lower than that with as-received Li anode, while values of ΔE1 of both cells are almost the same, indicating that the electrochemical reaction of lithium sulfide and polysulfides becomes easier owning to the high Li ion conductivity of protective film. Moreover, Fig. 5(d) displays the variation of total integrated areas of reduction peaks (ARE) and oxidation peaks (AOX) with cycle number, respectively. ARE and AOX are associated with the discharge and charge capacity of the cell, and the ratio of ARE/AOX is related to cycling efficiency. As seen, both ARE and AOX of the cell with modified Li electrode is larger than that with as-received Li anode, indicating that the energy density of Li–S cell is improved, owning to the effective suppression of Li2S2/Li2S corrosion and high Li ion conductivity of the protective film by the surface modification. In addition, the ratio of ARE/AOX is almost 1 for all CV curves except the first scan of Li–S cell with modified Li anode, indicating high cycling efficiency of both cells after the first cycle. However, ARE and AOX of both cells decrease very fast with cycle number owing to the excess dissolution of lithium polysulfides into electrolyte,49 which also corresponds with the results of cycling performance tests in Fig. 6(a).
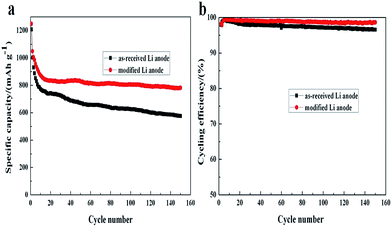 | ||
| Fig. 6 The long-term cycling performances of Li–S batteries at 0.2C with different Li anodes: specific capacity vs. cycle number (a), and cycling efficiency vs. cycle number (b), respectively. | ||
Capacity retention and cycling efficiency are two important parameters to evaluate the cell performance. Fig. 6(a) and (b) present the discharge specific capacity and cycling efficiency of Li/S cells with different Li anodes over 150 cycles at 0.2C, respectively. As seen, the capacity of both cells fade rapidly during the first 10 cycles with a capacity loss of ca. 400 mA h g−1, which is consist with the results in Fig. 5(d) owing the high solubility of lithium polysulfides. However, for the cell with as-received Li anode, its specific capacity decreases to 574 mA h g−1 after 150 cycles with capacity retention of only 47.5%. Moreover, the cycling efficiency gradually decreases even with 1 wt% LiNO3 addition in the electrolyte. In contrast, although the capacity degradation is relatively fast in the first 10 cycles with the modification, it tends to stabilize at about 800 mA h g−1 after that and keeps a capacity retention of ∼68%, indicating a good cycling performance. And also, a high cycling efficiency over 98.5% is maintained for 150 cycles. It can be ascribed to the denser and more conductive SEI layer on modified Li anode surface, which has effectively prevented Li anode from lithium sulfides corrosion and improved utilization of sulfur.
The morphologies and EDS maps of two different Li anodes from the cycled Li/S cells were compared in Fig. 7. The cycled as-received Li anode shows a rough and irregular morphology with many cracks and holes on the surface. Thus, it is convenient for the lithium sulfides to diffuse through creaks and holes, causing severe corrosion of Li anode after repeated cycles, which can be demonstrated by the cross-section image in Fig. 7(c). Moreover, the presence of high-content S in the EDS spectrum of Fig. 7(e) indicates that many S-containing species have heterogeneously deposited on as-received Li anode surface, which will block ions transfer and increase polarization potential, and even causes the failure of the cell.50,51
However, the modified Li electrode exhibits a denser and smoother surface film with large bulk solids closely packed together (Fig. 7(b)). The cross-section image of modified Li anode in Fig. 7(d) displays a intact bulk structure after 150 cycles, indicating less corrosion appeared on the surface, which can also be confirmed by less amount of S-containing species detected from EDS map in Fig. 7(f). In a summary, the protection of Li anode has effectively blocked the access of polysulfides to the Li surface and side reactions between them, restraining continuous Li corrosion, meanwhile reducing irreversible precipitates.
The stability of the protective film during the charge/discharge cycling tests was investigated by XPS. Fig. 8(a) shows the XPS results of the cycled Li anode surface with (CH3)3SnSn(CH3)3 modification after 20 s etching. According to the reaction scheme proposed by Aurbach52 and Xiong,24 the composition in SEI layer of Li anode cycled in LiTFSI electrolyte with LiNO3 additive is mainly composed of LiyC2Fx, LiF, Li2S2O4, Li2S Li2NSO2CF3, LiNxOy, Li3N, etc., which are resulted from reduction products of TFSI− anion, polysulfides and LiNO3. Fig. 8(b) presents a magnified XPS spectrum of Sn 3d. Two strong peaks at 485.9 eV/494.2 eV with two tiny peaks at around 483.2 eV/491.2 eV can be attributed to the formation of Sn–C and Sn–Li, respectively, resulting from the formation of (CH3)3SnLi as evidenced by Fig. 2. Hence, it can be indicated that the protective film is very stable in the electrolyte during long-term cycling.
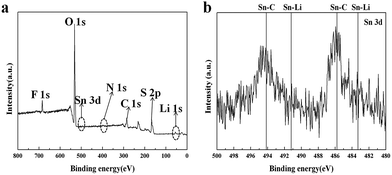 | ||
| Fig. 8 XPS analysis of the modified Li anode surface from Li/S cell after 150 cycles (a) with magnified XPS spectrum of the Sn 3d region (b). | ||
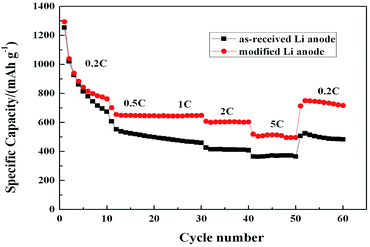
The rate capability is an important performance parameter of Li–S batteries especially when they are applied in an occasion of high power supply. Fig. 9 shows the rate capability behaviors of the Li/S cells with different Li anodes in the current density ranging from 0.2C to 5C. As seen, both cells with different Li anodes have comparable capacity during the first 5 cycles at 0.2C owing to the formation and growth of SEI layer by the addition of LiNO3. However, as the current density increases, the reversible capacity of the cell with modified Li anode is obviously higher than that with as-received Li anode especially at high rates, which exhibits an impressive specific capacity of 510 mA h g−1 at 5C. It is likely attributed to less corrosion of lithium sulfides and high utilization ratio of active S by the surface modification of Li anode.6 Noticeably, the cell with modified Li anode shows a tiny gap of specific capacity between 0.5C and 1C (ca. 4 mA h g−1), resulting from largely decreased overpotential and greatly increased utilization ratio of active S at high current density through surface modification of Li anode. When the current density is switch abruptly from 5C to 0.2C, a stable capacity of ∼800 mA h g−1 is recovered in the cell with modified Li anode after 60 cycles, while the cell with as-received Li anode has gradually decreased to 500 mA h g−1, which agrees with the results of Fig. 6(b). It can be indicated that the protective film on modified Li anode surface has remarkable stability in high-power working conditions. In a word, the rate capability of Li–S is greatly improved by surface modification of Li anode.
Conclusions
In order to prevent Li electrode corrosion in the presence of sulfur-containing species and other electrolyte components, a protective film was formed on Li electrode surface by hexamethylditin modification. The modified Li electrode exhibits higher stability, lower interface resistance and lower charge-transfer resistance as well in the electrolyte. Moreover, the modified Li electrode performs stable Li+ plating/striping behavior with a lower polarization potential difference over 100 cycles, while the as-received Li anode shows unstable polarization as confirmed by the large and frustrated interface resistance of as-received Li electrode during cycling, indicating that the protective film on modified Li electrode is more efficient than the SEI film formed by LiNO3 additive in electrolyte. Furthermore, the Li–S cell with modified Li anode exhibits a greatly improved cycling performance with a stable discharge capacity of about 800 mA h g−1 after 150 cycles at 0.2C and an impressive cycling efficiency of over 98.5%, which is much better than that with as-received Li anode. In addition, the rate performance of Li–S cell is also significantly enhanced by the surface modification, delivering a specific capacity of 510 mA h g−1 at 5C, which will make it more competitive in high power supply market.Acknowledgements
This work was financially supported by NSFC Project No. 51373195 and No. 51201177; research projects from the Science and Technology Commission of Shanghai Municipality No. 14JC1493000 and No. 15DZ2281200.Notes and references
- J. Liu, J. G. Zhang, Z. G. Yang, J. P. Lemmon, C. Imhoff, G. L. Graff, L. Y. Li, J. Z. Hu, C. M. Wang, J. Xiao, G. Xia, V. V. Viswanathan, S. Baskaran, V. Sprenkle, X. L. Li, Y. Y. Shao and B. Schwenzer, Adv. Funct. Mater., 2013, 23, 929–946 CrossRef CAS.
- X. L. Ji and L. F. Nazar, J. Mater. Chem., 2010, 20, 9821–9826 RSC.
- Z. Lin and C. D. Liang, J. Mater. Chem. A, 2015, 3, 936–958 CAS.
- P. G. Bruce, L. J. Hardwick and K. M. Abraham, MRS Bull., 2011, 36, 506–512 CrossRef CAS.
- S. Evers and L. F. Nazar, Acc. Chem. Res., 2013, 46, 1135–1143 CrossRef CAS PubMed.
- S. E. Cheon, K. S. Ko, J. H. Cho, S. W. Kim, E. Y. Chin and H. T. Kim, J. Electrochem. Soc., 2003, 150, A800–A805 CrossRef CAS.
- J. Shim, K. A. Striebel and E. J. Cairns, J. Electrochem. Soc., 2002, 149, A1321–A1325 CrossRef CAS.
- D.-I. S. V. Knapa, M. Swierczynskia, R. Teodorescua and E. Schaltza, ECS Trans., 2015, 70, 95–103 CrossRef.
- H. S. Ryu, H. J. Ahn, K. W. Kim, J. H. Ahn, J. Y. Lee and E. J. Cairns, J. Power Sources, 2005, 140, 365–369 CrossRef CAS.
- H. S. Ryu, H. J. Ahn, K. W. Kim, J. H. Ahn, K. K. Cho and T. H. Nam, Electrochim. Acta, 2006, 52, 1563–1566 CrossRef CAS.
- J. Schuster, G. He, B. Mandlmeier, T. Yim, K. T. Lee, T. Bein and L. F. Nazar, Angew. Chem., Int. Ed., 2012, 51, 3591–3595 CrossRef CAS PubMed.
- A. Manthiram, Y. Z. Fu, S. H. Chung, C. X. Zu and Y. S. Su, Chem. Rev., 2014, 114, 11751–11787 CrossRef CAS PubMed.
- Q. Pang, D. Kundu, M. Cuisinier and L. F. Nazar, Nat. Commun., 2014, 5 Search PubMed.
- Y. V. Mikhaylik and J. R. Akridge, J. Electrochem. Soc., 2004, 151, A1969–A1976 CrossRef CAS.
- A. Manthiram, Y. Z. Fu and Y. S. Su, Acc. Chem. Res., 2013, 46, 1125–1134 CrossRef CAS PubMed.
- K. M. A. R. D. Rauh, G. F. Pearson and S. B. Brummer, J. Electrochem. Soc., 1979, 126, 523–527 CrossRef.
- E. Peled, Y. Sternberg, A. Gorenshtein and Y. Lavi, J. Electrochem. Soc., 1989, 136, 1621–1625 CrossRef CAS.
- D. Aurbach, E. Pollak, R. Elazari, G. Salitra, C. S. Kelley and J. Affinito, J. Electrochem. Soc., 2009, 156, A694–A702 CrossRef CAS.
- I. K. Yuriy, V. Mikhaylik, R. Schock, K. Kumaresan, J. Xu and J. Affinito, ECS Trans., 2010, 25, 23–34 Search PubMed.
- K. Xu, Chem. Rev., 2004, 104, 4303–4417 CrossRef CAS PubMed.
- Y. X. Yin, S. Xin, Y. G. Guo and L. J. Wan, Angew. Chem., Int. Ed., 2013, 52, 13186–13200 CrossRef CAS PubMed.
- S. S. Zhang, J. Electrochem. Soc., 2012, 159, A920–A923 CrossRef CAS.
- S. S. Zhang, Electrochim. Acta, 2012, 70, 344–348 CrossRef CAS.
- S. Z. Xiong, K. Xie, Y. Diao and X. B. Hong, Electrochim. Acta, 2012, 83, 78–86 CrossRef CAS.
- X. Liang, Z. Y. Wen, Y. Liu, M. F. Wu, J. Jin, H. Zhang and X. W. Wu, J. Power Sources, 2011, 196, 9839–9843 CrossRef CAS.
- Z. Lin, Z. C. Liu, W. J. Fu, N. J. Dudney and C. D. Liang, Adv. Funct. Mater., 2013, 23, 1064–1069 CrossRef CAS.
- J. C. Burns, R. Petibon, K. J. Nelson, N. N. Sinha, A. Kassam, B. M. Way and J. R. Dahna, J. Electrochem. Soc., 2013, 160, A1668–A1674 CrossRef CAS.
- F. Ding, W. Xu, G. L. Graff, J. Zhang, M. L. Sushko, X. L. Chen, Y. Y. Shao, M. H. Engelhard, Z. M. Nie, J. Xiao, X. J. Liu, P. V. Sushko, J. Liu and J. G. Zhang, J. Am. Chem. Soc., 2013, 135, 4450–4456 CrossRef CAS PubMed.
- H. Kim, J. T. Lee, D. C. Lee, M. Oschatz, W. I. Cho, S. Kaskel and G. Yushin, Electrochem. Commun., 2013, 36, 38–41 CrossRef CAS.
- Y. M. Lee, N. S. Choi, J. H. Park and J. K. Park, J. Power Sources, 2003, 119, 964–972 CrossRef.
- G. Q. Ma, Z. Y. Wen, Q. S. Wang, C. Shen, J. Jin and X. W. Wu, J. Mater. Chem. A, 2014, 2, 19355–19359 CAS.
- Y.-S. L. Ik Su Kang and D.-W. Kim, J. Electrochem. Soc., 2014, 161(1), A53–A57 CrossRef.
- G. Q. Ma, Z. Y. Wen, M. F. Wu, C. Shen, Q. S. Wang, J. Jin and X. W. Wu, Chem. Commun., 2014, 50, 14209–14212 RSC.
- D. J. Lee, H. Lee, J. Song, M. H. Ryou, Y. M. Lee, H. T. Kim and J. K. Park, Electrochem. Commun., 2014, 40, 45–48 CrossRef CAS.
- X. L. Zhang, W. K. Wang, A. B. Wang, Y. Q. Huang, K. G. Yuan, Z. B. Yu, J. Y. Qiu and Y. S. Yang, J. Mater. Chem. A, 2014, 2, 11660–11665 CAS.
- X. B. Cheng, H. J. Peng, J. Q. Huang, F. Wei and Q. Zhang, Small, 2014, 10, 4257–4263 CAS.
- D. Y. Wang, C. Wang and M. Uchiyama, J. Am. Chem. Soc., 2015, 137, 10488–10491 CAS.
- K. Kanamura, S. Shiraishi, H. Tamura and Z. Takehara, J. Electrochem. Soc., 1994, 141, 2379–2385 CrossRef CAS.
- W. M.-F. W. Z.-Y. L. Yu, Acta Phys.-Chim. Sin., 2011, 27, 1695–1700 Search PubMed.
- M. Yus, Chem. Soc. Rev., 1996, 25, 155–161 RSC.
- D. J. Ramon and M. Yus, J. Org. Chem., 1991, 56, 3825–3831 CrossRef CAS.
- J. Smid, J. Am. Chem. Soc., 1965, 87, 655–656 CrossRef CAS.
- N. L. Holy, Chem. Rev., 1974, 74, 244–277 CrossRef.
- M. Yus, R. P. Herrera and A. Guijarro, Tetrahedron Lett., 2001, 42, 3455–3458 CrossRef CAS.
- S. M. Choi, I. S. Kang, Y. K. Sun, J. H. Song, S. M. Chung and D. W. Kim, J. Power Sources, 2013, 244, 363–368 CrossRef CAS.
- D. Aurbach, E. Zinigrad, Y. Cohen and H. Teller, Solid State Ionics, 2002, 148, 405–416 CrossRef CAS.
- J. C. Guo, Y. H. Xu and C. S. Wang, Nano Lett., 2011, 11, 4288–4294 CrossRef CAS PubMed.
- H. Yamin, A. Gorenshtein, J. Penciner, Y. Sternberg and E. Peled, J. Electrochem. Soc., 1988, 135, 1045–1048 CrossRef CAS.
- G. Y. Zheng, Q. F. Zhang, J. J. Cha, Y. Yang, W. Y. Li, Z. W. Seh and Y. Cui, Nano Lett., 2013, 13, 1265–1270 CrossRef CAS PubMed.
- J. M. Zheng, M. Gu, H. H. Chen, P. Meduri, M. H. Engelhard, J. G. Zhang, J. Liu and J. Xiao, J. Mater. Chem. A, 2013, 1, 8464–8470 CAS.
- J. Q. Huang, Q. Zhang, H. J. Peng, X. Y. Liu, W. Z. Qian and F. Wei, Energy Environ. Sci., 2014, 7, 347–353 CAS.
- D. Aurbach, I. Weissman, A. Zaban and O. Chusid, Electrochim. Acta, 1994, 39, 51–71 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |