Reaction control in heterogeneous catalysis using montmorillonite: switching between acid-catalysed and red-ox processes†
Abstract
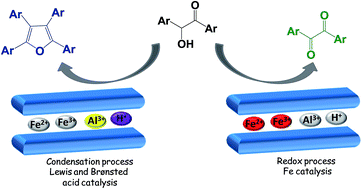
The use of montmorillonite, modified with a super-acid (CF3SO3H), in the presence of hydroquinone as a radical scavenger and under a nitrogen atmosphere, induced the formation of tetrasubstituted furans as the major product from benzoins. In the absence of a radical scavenger, the only products obtained were 1,2-diketones.

 Please wait while we load your content...
Please wait while we load your content...